Biomarkers for Exposure as a Tool for Efficacy Testing of a Mycotoxin Detoxifier in Broiler Chickens and Pigs
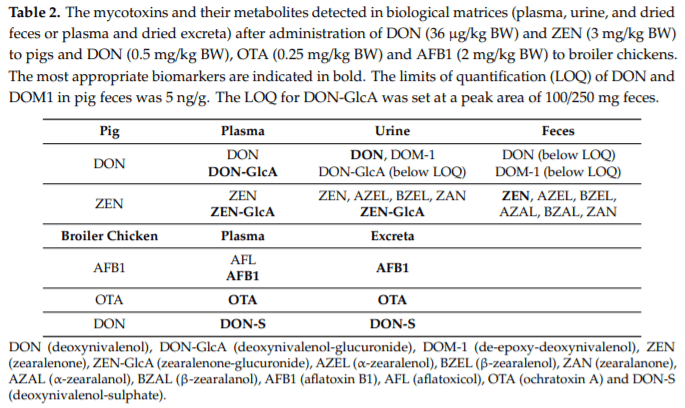
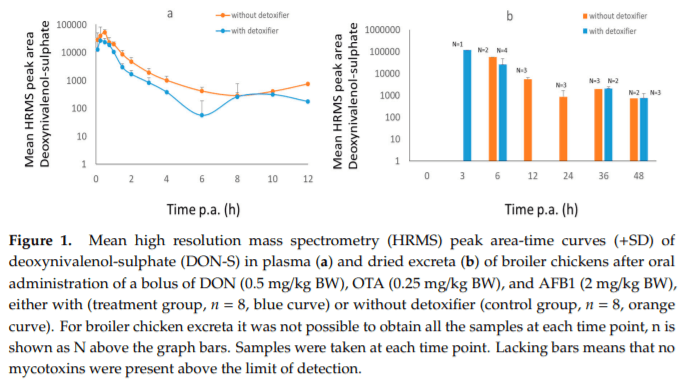
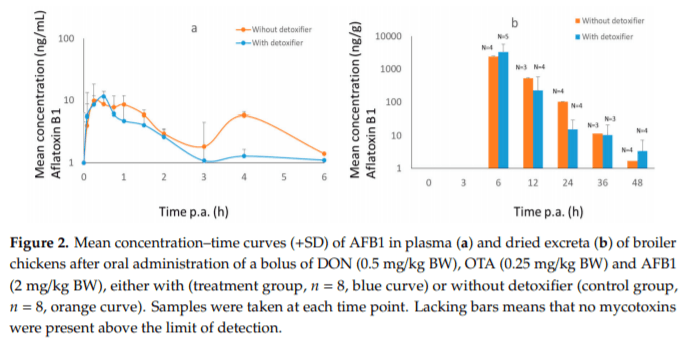
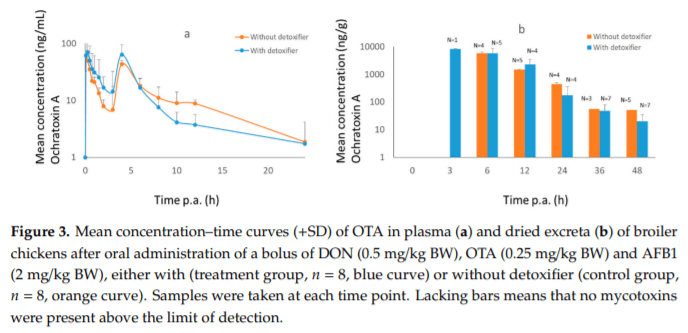
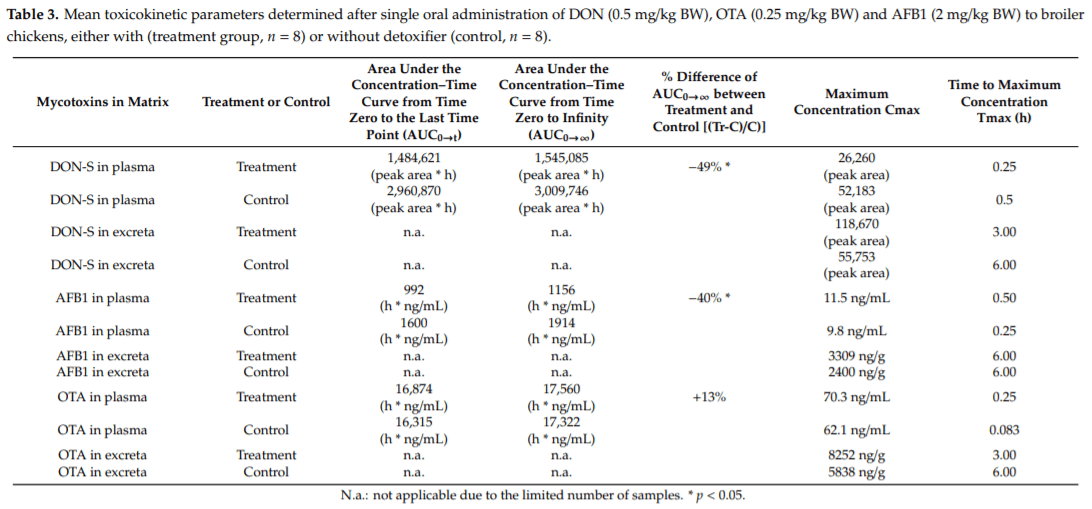
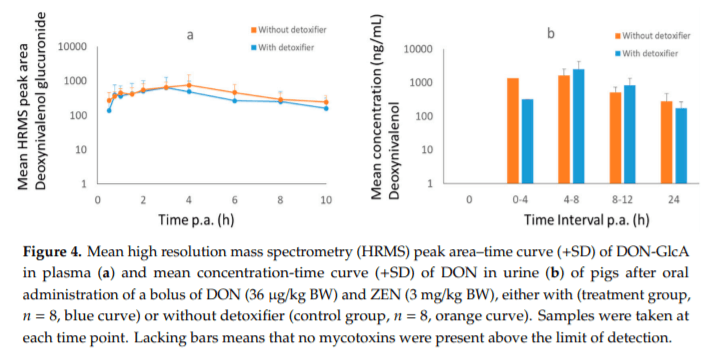
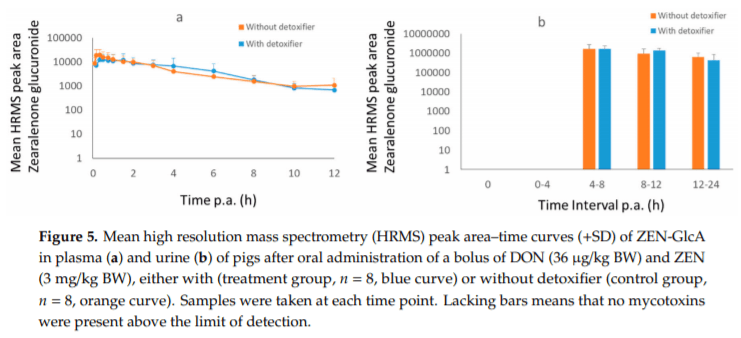
Applying post-harvest control measures such as adding mycotoxin detoxifying agents is a frequently-used mitigation strategy for mycotoxins. EFSA states that the efficacy of these detoxifiers needs to be tested using specific biomarkers for exposure. However, the proposed biomarkers for exposure are not further optimized for specific target species. Hence, the goal of this study was (a) to evaluate the most suitable biomarkers for deoxynivalenol (DON) and zearalenone (ZEN) in porcine plasma, urine and feces; and DON, aflatoxin B1 (AFB1) and ochratoxin A (OTA) in plasma and excreta of broiler chickens and (b) to determine the efficacy of a candidate detoxifier, as a proof-of-concept study. Therefore, a mixture of mycotoxins was administered as a single oral bolus with or without detoxifying agent. In accordance with literature AFB1, OTA, and DON-sulphate (DON-S) proved optimal biomarkers in broilers plasma and excreta whereas, in pigs DON-glucuronide (DON-GlcA) and ZEN-glucuronide (ZEN-GlcA) proved the optimal biomarkers in plasma, DON and ZEN-GlcA in urine and, ZEN in feces. A statistically significant reduction was seen between control and treatment group for both AFB1 and DON in broiler plasma, under administration of the mycotoxin blend and detoxifier dose studied suggesting thus, beneficial bioactivity.
Keywords: biomarkers; exposure; efficacy; mycotoxin detoxifier; pig; broiler chicken
Key Contribution: Optimal biomarkers for exposure were determined for DON and ZEN in porcine plasma, urine, and feces; and DON, AFB1, and OTA in plasma and excreta of broiler chickens. These biomarkers were successfully applied to determine the efficacy of a candidate mycotoxin detoxifier.
1. Kongkapan, J.; Poapolathep, S.; Isariyodom, S.; Kumagai, S.; Poapolathep, A. Simultaneous detection of multiple mycotoxins in broiler feeds using a liquid chromatography tandem-mass spectrometry. J. Vet. Med. Sci. 2016, 78, 259–264. [CrossRef]
2. Rawal, S.; Kim, J.E.; Coulombe, R. Aflatoxin B1 in poultry: Toxicology, metabolism and prevention. Res. Vet. Sci. 2010, 89, 325–331. [CrossRef]
3. Khatoon, A.; Khan, M.Z.; Abidin, Z.; Bhatti, S.A. Effects of feeding bentonite clay upon ochratoxin A-induced immunosuppression in broiler chicks. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2018, 35, 538–545. [CrossRef] [PubMed]
4. Pfohl-Leszkowicz, A.; Manderville, R.A. Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans. Mol. Nutr. Food Res. 2007, 51, 61–99. [CrossRef] [PubMed]
5. Kovalsky, P.; Kos, G.; Nährer, K.; Schwab, C.; Jenkins, T.; Schatzmayr, G.; Sulyok, M.; Krska, R. Co-occurrence of regulated, masked and emerging mycotoxins and secondary metabolites in finished feed and maize–An extensive survey. Toxins 2016, 8, 363. [CrossRef] [PubMed]
6. Yu, Y.H.; Hsiao, F.S.H.; Proskura, W.S.; Dybus, A.; Siao, Y.H.; Cheng, Y.H. An impact of Deoxynivalenol produced by Fusarium graminearum on broiler chickens. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1012–1019. [CrossRef]
7. Döll, S.; Dänicke, S. The Fusarium toxins deoxynivalenol (DON) and zearalenone (ZON) in animal feeding. Prev. Vet. Med. 2011, 102, 132–145. [CrossRef] [PubMed]
8. Awad, W.A.; Ghareeb, K.; Böhm, J.; Zentek, J. Decontamination and detoxification strategies for the Fusarium mycotoxin deoxynivalenol in animal feed and the effectiveness of microbial biodegradation. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2010, 27, 510–520. [CrossRef] [PubMed]
9. Pappas, A.C.; Tsiplakou, E.; Tsitsigiannis, D.I.; Georgiadou, M.; Iliadi, M.K.; Sotirakoglou, K.; Zervas, G. The role of bentonite binders in single or concomitant mycotoxin contamination of chicken diets. Br. Poult. Sci. 2016, 57, 551–558. [CrossRef] [PubMed]
10. Pasha, T.N.; Farooq, M.U.; Khattak, F.M.; Jabbar, M.A.; Khan, A.D. Effectiveness of sodium bentonite and two commercial products as aflatoxin absorbents in diets for broiler chickens. Anim. Feed Sci. Technol. 2007, 132, 103–110. [CrossRef]
11. Bhatti, S.A.; Khan, M.Z.; Saleemi, M.K.; Saqib, M.; Khan, A.; Ul-Hassan, Z. Protective role of bentonite against aflatoxin B1- and ochratoxin A-induced immunotoxicity in broilers. J. Immunotoxicol. 2017, 14, 66–76. [CrossRef]
12. Miazzo, R.; Peralta, M.F.; Magnoli, C.; Salvano, M.; Ferrero, S.; Chiacchiera, S.M.; Carvalho, E.C.Q.; Rosa, C.A.R.; Dalcero, A. Efficacy of sodium bentonite as a detoxifier of broiler feed contaminated with aflatoxin and fumonisin. Poult. Sci. 2005, 84, 1–8. [CrossRef] [PubMed]
13. Neeff, D.V.; Ledoux, D.R.; Rottinghaus, G.E.; Bermudez, A.J.; Dakovic, A.; Murarolli, R.A.; Oliveira, C.A.F. In vitro and in vivo efficacy of a hydrated sodium calcium aluminosilicate to bind and reduce aflatoxin residues in tissues of broiler chicks fed aflatoxin B1. Poult. Sci. 2013, 92, 131–137. [CrossRef] [PubMed]
14. Eckhardt, J.C.; Santurio, J.M.; Zanette, R.A.; Rosa, A.P.; Scher, A.; Dal Pozzo, M.; Alves, S.H.; Ferreiro, L. Efficacy of a Brazilian calcium montmorillonite against toxic effects of dietary aflatoxins on broilers reared to market weight. Br. Poult. Sci. 2014, 55, 215–220. [CrossRef]
15. Magnoli, A.P.; Texeira, M.; Rosa, C.A.R.; Miazzo, R.D.; Cavaglieri, L.R.; Magnoli, C.E.; Dalcero, A.M.; Chiacchiera, S.M. Sodium bentonite and monensin under chronic aflatoxicosis in broiler chickens. Poult. Sci. 2011, 90, 352–357. [CrossRef]
16. Santurio, J.M.; Mallmann, C.A.; Rosa, A.P.; Appel, G.; Heer, A.; Dageförde, S.; Böttcher, M. Effect of sodium bentonite on the performance and blood variables of broiler chickens intoxicated with aflatoxins. Br. Poult. Sci. 1999, 40, 115–119. [CrossRef]
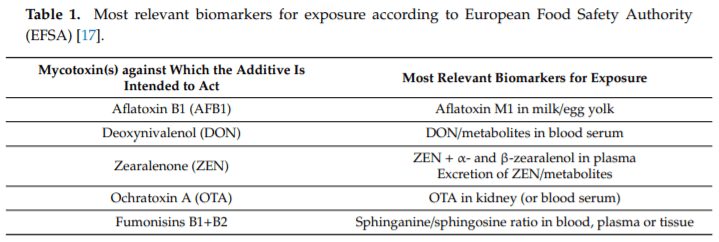
17. EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). EFSA Statement on the establishment of guidelines for the assessment of additives from the functional group ‘substances for reduction of the contamination of feed by mycotoxins’. EFSA J. 2010, 8, 1963. [CrossRef]
18. Lauwers, M.; De Baere, S.; Letor, B.; Rychlik, M.; Croubels, S.; Devreese, M. Multi LC-MS/MS and LC-HRMS Methods for Determination of 24 Mycotoxins including Major Phase I and II Biomarker Metabolites in Biological Matrices from Pigs and Broiler Chickens. Toxins 2019, 11, 171. [CrossRef] [PubMed]
19. Dohnal, V.; Wu, Q.; Ku?ca, K. Metabolism of aflatoxins: Key enzymes and interindividual as well as interspecies differences. Arch. Toxicol. 2014, 88, 1635–1644. [CrossRef] [PubMed]
20. Mughal, M.J.; Peng, X.; Kamboh, A.A.; Zhou, Y.; Fang, J. Aflatoxin B1 Induced Systemic Toxicity in Poultry and Rescue Effects of Selenium and Zinc. Biol. Trace Elem. Res. 2017, 178, 292–300. [CrossRef]
21. Vidal, A.; Mengelers, M.; Yang, S.; De Saeger, S.; De Boevre, M. Mycotoxin Biomarkers of Exposure: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1127–1155. [CrossRef]
22. Tao, Y.; Xie, S.; Xu, F.; Liu, A.; Wang, Y.; Chen, D.; Pan, Y.; Huang, L.; Peng, D.; Wang, X.; et al. Ochratoxin A: Toxicity, oxidative stress and metabolism. Food Chem. Toxicol. 2018, 112, 320–331. [CrossRef]
23. Yang, S.; Zhang, H.; De Saeger, S.; De Boevre, M.; Sun, F.; Zhang, S.; Cao, X.; Wang, Z. In vitro and in vivo metabolism of ochratoxin A: A comparative study using ultra-performance liquid chromatography-quadrupole/time-of-flight hybrid mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 3579–3589. [CrossRef]
24. Ringot, D.; Chango, A.; Schneider, Y.J.; Larondelle, Y. Toxicokinetics and toxicodynamics of ochratoxin A, an update. Chem. Biol. Interact. 2006, 159, 18–46. [CrossRef]
25. Dänicke, S.; Brezina, U. Kinetics and metabolism of the Fusarium toxin deoxynivalenol in farm animals: Consequences for diagnosis of exposure and intoxication and carry over. Food Chem. Toxicol. 2013, 60, 58–75. [CrossRef]
26. Valgaeren, B.; Théron, L.; Croubels, S.; Devreese, M.; De Baere, S.; Van Pame, E.; Daeseleire, L.; De Boevre, M.; De Saeger, S.; Vidal, A.; et al. The role of roughage provision on the absorption and disposition of the mycotoxin deoxynivalenol and its acetylated derivatives in calves: From field observations to toxicokinetics. Arch. Toxicol. 2018, 93, 293–310. [CrossRef]
27. Devreese, M.; Antonissen, G.; Broekaert, N.; De Mil, T.; De Baere, S.; Vanhaecke, L.; De Backer, P.; Croubels, S. Toxicokinetic study and oral bioavailability of deoxynivalenol in turkey poults, and comparative biotransformation between broilers and turkeys. World Mycotoxin J. 2015, 8, 533–539. [CrossRef]
28. Malekinejad, H.; Maas-Bakker, R.; Fink-Gremmels, J. Species differences in the hepatic biotransformation of zearalenone. Vet. J. 2006, 172, 96–102. [CrossRef]
29. Fleck, S.C.; Churchwell, M.I.; Doerge, D.R. Metabolism and pharmacokinetics of zearalenone following oral and intravenous administration in juvenile female pigs. Food Chem. Toxicol. 2017, 106, 193–201. [CrossRef]
30. Catteuw, A.; Broekaert, N.; De Baere, S.; Lauwers, M.; Gasthuys, E.; Huybrechts, B.; Callebaut, A.; Ivanova, L.; Uhlig, S.; De Boevre, M.; et al. Insights into In Vivo Absolute Oral Bioavailability, Biotransformation, and Toxicokinetics of Zearalenone, α-Zearalenol, β-Zearalenol, Zearalenone-14-glucoside, and Zearalenone-14-sulfate in Pigs. J. Agric. Food Chem. 2019. [CrossRef]
31. Broekaert, N.; Devreese, M.; van Bergen, T.; Schauvliege, S.; De Boevre, M.; De Saeger, S.; Vanhaecke, L.; Berthiller, F.; Michlmayr, H.; Malachová, A.; et al. In vivo contribution of deoxynivalenol-3-β-d-glucoside to deoxynivalenol exposure in broiler chickens and pigs: Oral bioavailability, hydrolysis and toxicokinetics. Arch. Toxicol. 2017, 91, 699–712. [CrossRef]
32. Devreese, M.; Antonissen, G.; Broekaert, N.; De Baere, S.; Vanhaecke, L.; De Backer, P.; Croubels, S. Comparative Toxicokinetics, Absolute Oral Bioavailability, and Biotransformation of Zearalenone in Different Poultry Species. J. Agric. Food Chem. 2015, 63, 5092–5098. [CrossRef]
33. Devreese, M.; Osselaere, A.; Goossens, J.; Vandenbroucke, V.; De Baere, S.; Eeckhout, M.; De Backer, P.; Croubels, S. New bolus models for in vivo efficacy testing of mycotoxin-detoxifying agents in relation to EFSA guidelines, assessed using deoxynivalenol in broiler chickens. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2012, 29, 1101–1107. [CrossRef]
34. Osselaere, A.; Devreese, M.; Watteyn, A.; Vandenbroucke, V.; Goossens, J.; Hautekiet, V.; Eeckhout, M.; De Saeger, S.; De Baere, S.; De Backer, P.; et al. Efficacy and safety testing of mycotoxin-detoxifying agents in broilers following the European Food Safety Authority guidelines. Poult. Sci. 2012, 91, 2046–2054. [CrossRef]
35. Di Gregorio, M.C.; Jager, A.V.; Souto, P.C.M.C.; Costa, A.A.; Rottinghaus, G.E.; Passarelli, D.; Budiño, F.E.L.; Corassin, C.H.; Oliveira, C.A.F. Determination of serum aflatoxin B1-lysine to evaluate the efficacy of an aflatoxin-adsorbing feed additive in pigs fed an aflatoxin B1-contaminated diet. Mycotoxin Res. 2017, 33, 93–102. [CrossRef]
36. Masching, S.; Naehrer, K.; Schwartz-Zimmermann, H.E.; Sarandan, M.; Schaumberger, S.; Dohnal, I.; Nagl, V.; Schatzmayr, D. Gastrointestinal degradation of fumonisin B1 by carboxylesterase FumD prevents fumonisin induced alteration of sphingolipid metabolism in Turkey and swine. Toxins 2016, 8, 84. [CrossRef]
37. Dänicke, S.; Ueberschär, K.-H.; Halle, I.; Valenta, H.; Flachowsky, G. Excretion kinetics and metabolism of zearalenone in broilers in dependence on a detoxifying agent. Arch. Anim. Nutr. 2001, 55, 299–313. [CrossRef]
38. Gambacorta, L.; Pinton, P.; Avantaggiato, G.; Oswald, I.P.; Solfrizzo, M. Grape Pomace, an Agricultural Byproduct Reducing Mycotoxin Absorption: In Vivo Assessment in Pig Using Urinary Biomarkers. J. Agric. Food Chem. 2016, 64, 6762–6771. [CrossRef]
39. Lozano, M.C.; Diaz, G.J. Microsomal and cytosolic biotransformation of aflatoxin B1 in four poultry species. Br. Poult. Sci. 2006, 47, 734–741. [CrossRef]
40. Di Gregorio, M.C.; Jager, A.V.; Costa, A.A.; Bordin, K.; Rottinhghaus, G.E.; Petta, T.; Souto, P.C.M.C.; Budiño, F.E.L.; Oliveira, C.A.F. Determination of Aflatoxin B1-Lysine in Pig Serum and Plasma by Liquid Chromatography—Tandem Mass Spectrometry. J. Anal. Toxicol. 2016, 41, 236–241. [CrossRef]
41. Leong, Y.H.; Latiff, A.A.; Ahmad, N.I.; Rosma, A. Exposure measurement of aflatoxins and aflatoxin metabolites in human body fluids. A short review. Mycotoxin Res. 2012, 28, 79–87. [CrossRef]
42. Cortés, G.; Carvajal, M.; Méndez-Ramírez, I.; Ávila-González, E.; Chilpa-Galván, N.; Castillo-Urueta, P.; Flores, C.M. Identification and quantification of aflatoxins and aflatoxicol from poultry feed and their recovery in poultry litter. Poult. Sci. 2010, 89, 993–1001. [CrossRef]
43. Devreese, M.; Croubels, S.; De Baere, S.; Gehring, R.; Antonissen, G. Comparative Toxicokinetics and Plasma Protein Binding of Ochratoxin A in Four Avian Species. Agric. Food Chem. 2018, 66, 2129–2135. [CrossRef]
44. Schwartz-Zimmermann, H.E.; Fruhmann, P.; Dänicke, S.; Wiesenberger, G.; Caha, S.; Weber, J.; Berthiller, F. Metabolism of deoxynivalenol and deepoxy-deoxynivalenol in broiler chickens, pullets, roosters and turkeys. Toxins 2015, 7, 4706–4729. [CrossRef]
45. Dänicke, S.; Valenta, H.; Döll, S. On the toxicokinetics and the metabolism of deoxynivalenol (don) in the pig. Arch. Anim. Nutr. 2007, 58, 169–180. [CrossRef]
46. Nagl, V.; Woechtl, B.; Schwartz-Zimmermann, H.E.; Hennig-Pauka, I.; Moll, W.D.; Adam, G.; Berthiller, F. Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in pigs. Toxicol. Lett. 2014, 229, 190–197. [CrossRef]
47. Goyarts, T.; Dänicke, S. Bioavailability of the Fusarium toxin deoxynivalenol (DON) from naturally contaminated wheat for the pig. Toxicol. Lett. 2006, 163, 171–182. [CrossRef]
48. De Baere, S.; Osselaere, A.; Devreese, M.; Vanhaecke, L.; De Backer, P.; Croubels, S. Development of a liquid-chromatography tandem mass spectrometry and ultra-high-performance liquid chromatography high-resolution mass spectrometry method for the quantitative determination of zearalenone and its major metabolites in chicken and pig plasma. Anal. Chim. Acta 2012, 756, 37–48. [CrossRef]
49. Binder, S.B.; Schwartz-Zimmermann, H.E.; Varga, E.; Bichl, G.; Michlmayr, H.; Adam, G.; Berthiller, F. Metabolism of zearalenone and its major modified forms in pigs. Toxins 2017, 9, 56. [CrossRef]
50. Antonissen, G.; Martel, A.; Pasmans, F.; Ducatelle, R.; Verbrugghe, E.; Vandenbroucke, V.; Li, S.; Haesebrouck, F.; Van Immerseel, F.; Croubels, S. The impact of Fusarium Mycotoxins on human and animal host susceptibility to infectious diseases. Toxins 2014, 6, 430–452. [CrossRef]
51. Miazzo, R.; Rosa, C.A.R.; De Queiroz Carvalho, E.C.; Magnoli, C.; Chiacchiera, S.M.; Palacio, G.; Saenz, M.; Kikot, A.; Basaldella, E.; Dalcero, A. Efficacy of synthetic zeolite to reduce the toxicity of aflatoxin in broiler chicks. Poult. Sci. 2000, 79, 1–6. [CrossRef]
52. Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; de Lourdes Bastos, M.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; Gropp, J.; et al. Safety and efficacy of bentonite as a feed additive for all animal species. EFSA J. 2017, 15, 5096. [CrossRef]
53. Jiang, S.Z.; Yang, Z.B.; Yang, W.R.; Wang, S.J.; Wang, Y.; Broomhead, J.; Johnston, S.L.; Chi, F. Effect on hepatonephric organs, serum metabolites and oxidative stress in post-weaning piglets fed purified zearalenone-contaminated diets with or without Calibrin-Z. J. Anim. Physiol. Anim. Nutr. 2012, 96, 1147–1156. [CrossRef]
54. Jin, L.; Wang, W.; Degroote, J.; Van Noten, N.; Yan, H.; Majdeddin, M.; Van Poucke, M.; Peelman, L.; Goderis, A.; Van De Mierop, K.; et al. Mycotoxin binder improves growth rate in piglets associated with reduction of toll-like receptor-4 and increase of tight junction protein gene expression in gut mucosa. J. Anim. Sci. Biotechnol. 2017, 8, 80. [CrossRef]
55. Shehata, S.; Richter, W.; Schuster, M.; Lindermayer, H. Effect of Deoxynivalenol (DON) on growing pigs and its modification by modified yeast cell wall or modified yeast cell wall and bentonite. Mycotoxin Res. 2004, 20, 42–48. [CrossRef]
56. Frobose, H.L.; Erceg, J.A.; Fowler, S.Q.; Tokach, M.D.; DeRouchey, J.M.; Woodworth, J.C.; Dritz, S.S.; Goodband, R.D. The progression of deoxynivalenol-induced growth suppression in nursery pigs and the potential of an algae-modified montmorillonite clay to mitigate these effects1,2. J. Anim. Sci. 2016, 94, 3746–3759. [CrossRef]
57. Döll, S.; Gericke, S.; Dänicke, S.; Raila, J.; Ueberschär, K.H.; Valenta, H.; Schnurrbusch, U.; Schweigert, F.J.; Flachowsky, G. The efficacy of a modified aluminosilicate as a detoxifying agent in Fusarium toxin contaminated maize containing diets for piglets. J. Anim. Physiol. Anim. Nutr. 2005, 89, 342–358. [CrossRef]
58. Gasthuys, E.; Schauvliege, S.; van Bergen, T.; Millecam, J.; Cerasoli, I.; Martens, A.; Gasthuys, F.; Vandecasteele, T.; Cornillie, P.; Van den Broeck, W.; et al. Repetitive urine and blood sampling in neonatal and weaned piglets for pharmacokinetic and pharmacodynamic modelling in drug discovery: A pilot study. Lab. Anim. 2017, 51, 498–508. [CrossRef]
59. Heitzman, R. Veterinary Drug Residues: Residues in Food Producing Animals and Their Products: Reference Materials and Methods; Blackwell Scientific Publication: Oxford, UK, 1996; Volume 636, ISBN 0-8412-3419-1.
60. EMA/CMVP/VICH Steering Committee. VICH Topic GL49: Studies to Evaluate the Metabolism and Residues Kinetics of Veterinary Drugs in Human Food-Producing Animals: Validation of Analytical Methods Used in Residue Depletion Studies; European Medicine Agency: Brussels, Belgium, 2016; Volume 44.
61. European Commission. COMMISSION DECISION 2002/657/Implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Communities 2002, L221, 29. [CrossRef]









