Fumonisins in corn-based food
Mycotoxins in Corn-Based Food Products Consumed in Brazil: An Exposure Assessment for Fumonisins
Published: November 7, 2011
By: ELOISA D. CALDAS*, AND ANGELA C. S. SILVA (University of Brasília)
Samples from 10 different corn-based food products commercially sold in the Federal District of Brazil were analyzed for fumonisins (FB1 and FB2) using HPLC/fluorescence following naphthalene-2,3 dicarboxaldehyde (NDA) derivatization (limit of quantification (LOQ) ) 0.020 mg/kg). Samples were also analyzed for aflatoxins (B1, B2, G1, and G2) on a thin-layer chromatrography (TLC) plate under UV light (LOQ of 2 μg/kg). From the 208 samples analyzed, 80.7 and 71.6% had quantifiable levels of FB1 and FB2, respectively. Mean levels of total fumonisins (FB1 + FB2) ranged from 0.127 mg/kg for corn flakes to 2.04 mg/kg for cornmeal (creme de milho). No FBs were detected in any of the fresh, sweet corn on the cob samples analyzed. Aflatoxins were not detected in any of the 101 samples analyzed. The daily intakes of fumonisins through the consumption of corn-based food products was estimated using consumption data estimated from the 2002/2003 Brazilian Household Budget Survey and the level of fumonisins found in this and other studies conducted in Brazil. In the Federal District, the calculated total daily intake for the total and the consumers-only populations represented, respectively, 9.0 and 159% of the provisional maximum total daily intake (PMTDI) of 2 μg/kg body weight per day. At the national level, the intakes were calculated based on the fumonisin levels found in the Federal District and on published data from studies conducted elsewhere in the country. They represented 24.1 and 355% PMTDI for the total and the consumers-only populations, respectively. The high incidence of fumonisins in some corn-based products and the exposure levels found for specific subpopulations in the present study indicate the need for setting safe regulatory levels for fumonisins in food in Brazil.
KEYWORDS: Fumonisins; food corn products; daily intake; aflatoxins
INTRODUCTION
Fumonisins (FBs) are a group of structurally related mycotoxins produced mainly by Fusarium Verticillioides (Sacc.) Nirenberg () Fusarium moniliforme), which is one of the most prevalent fungi associated with maize grain (1, 2). Maize (corn) is the primary commodity contaminated with FBs, and fumonisin B1 (FB1), fumonisin B2 (FB2), and fumonisin B3 (FB3) have been previously reported in maize grain used for feed and food products worldwide (2, 3). Human exposure to fumonisincontaminated food has been linked to esophageal and liver cancer in South Africa and China (4, 5) and with neural tube defects in the United States (6). FB1 is classified as a possible human carcinogen (7). Aflatoxins (AFs) are mycotoxins produced by Aspergillus species that grow in many cereals and oilseeds but are found primarily in maize and peanuts (8, 9). Hepatocellular carcinoma resulting from chronic aflatoxin exposure is well-documented, and naturally occurring mixtures of aflatoxins and aflatoxin B1 (AFB1) are classified as human carcinogens (7).
The tropical and subtropical climates in Brazil favor fungal growth. Mycotoxin-contaminated grain or food, including aflatoxin and fumonisins in corn and corn-based food products, has been frequently reported (8, 10-12). The levels of fumonisins in corn-based food products are dependent upon the initial degree of grain contamination and the processing procedure used in making the final product, which may include degermination, nixtamalization, extrusion, milling, and the addition of ingredients such as other cereal grains and sugar (1, 13). Fumonisins are heat stable and survive under most conditions used during baking or frying (14, 15).
Human chronic exposure assessment data on fumonisins from the consumption of corn-based food products have been generated by studies conducted in multiple countries (16-19) and examined at the international level by the Joint FAO/WHO Expert Committee on Food Additives and Contaminants (JECFA) (20). These studies are important in defining safe regulatory levels of mycotoxin contamination in the food supply and in setting priorities for monitoring programs (20). Previous studies conducted in Brazil have assessed the exposure of the Brazilian population to fumonisins through the consumption of cornmeal (21-23), but the country still lacks an assessment that includes fumonisin intake from the consumption of all cornbased food products commonly found in the diet. Additionally, an assessment using data reflecting the level of fumonisins present in the food supply at the national level is still necessary.
Table 1. Sample Extraction and Cleanup on SAX Column
The tropical and subtropical climates in Brazil favor fungal growth. Mycotoxin-contaminated grain or food, including aflatoxin and fumonisins in corn and corn-based food products, has been frequently reported (8, 10-12). The levels of fumonisins in corn-based food products are dependent upon the initial degree of grain contamination and the processing procedure used in making the final product, which may include degermination, nixtamalization, extrusion, milling, and the addition of ingredients such as other cereal grains and sugar (1, 13). Fumonisins are heat stable and survive under most conditions used during baking or frying (14, 15).
Human chronic exposure assessment data on fumonisins from the consumption of corn-based food products have been generated by studies conducted in multiple countries (16-19) and examined at the international level by the Joint FAO/WHO Expert Committee on Food Additives and Contaminants (JECFA) (20). These studies are important in defining safe regulatory levels of mycotoxin contamination in the food supply and in setting priorities for monitoring programs (20). Previous studies conducted in Brazil have assessed the exposure of the Brazilian population to fumonisins through the consumption of cornmeal (21-23), but the country still lacks an assessment that includes fumonisin intake from the consumption of all cornbased food products commonly found in the diet. Additionally, an assessment using data reflecting the level of fumonisins present in the food supply at the national level is still necessary.
Table 1. Sample Extraction and Cleanup on SAX Column
The objectives of this study were to evaluate the level of fumonisins and aflatoxins in corn-based food products consumed within the Federal District, located in the Central West region of Brazil, and to evaluate the exposure levels and the potential health risks to the broader Brazilian population.
MATERIALS AND METHODS
Samples. A total of 208 samples (1 kg minimum per sample) of corn-based food products were collected or purchased from March 2003 to January 2005 at local retail stores in the Federal District. Products included fubá (cornmeal ) CM I), creme de milho (cornmeal ) CM II), popcorn, beiju (precooked corn flour ) PCF I), milharina (precooked corn flour ) PCF II), corn flakes, corn snacks, and sweet corn (fresh on the cob, frozen, and canned). Samples were stored in the laboratory at ambient temperature (20-30 °C), in the refrigerator (fresh corn on the cob), or in the freezer (frozen sweet corn) and were analyzed within 1 month of the collection date.
Standards and Reagent. Fumonisin (B1 and B2) and aflatoxin (B1, B2, G1, and G2) standards were purchased from Sigma-Aldrich (St. Louis, Mo). Stock solutions (1 mg/mL) of FB1 and FB2 were prepared in acetonitrile:water (1:1) and subsequently used to prepare a mixed solution containing 0.05 μg/mL of each fumonisin. Stock solutions (1 mg/mL) of each aflatoxin were prepared in benzene:acetonitrile (1:9 v/v) and used to prepare a mixed solution containing each aflatoxin at a 1 μg/mL final concentration. Acetonitrile (ACN) used was HPLC grade, and all other reagents and solvents were of analytical grade.
Fumonisin Analysis. Samples were analyzed using previously published methods (24, 25), with some modification. Samples were extracted for 30 min on a laboratory shaker using different solvent systems based on the food product being analyzed (Table 1). The extracts were filtered through qualitative Whatman filter paper, and 10 mL of the filtrate was added to a strong anion-exchange (SAX) column (Applied Separations, Allentown, PA) previously conditioned with 5 mL MeOH and 5 mL MeOH:water (1:1 v/v). The column was washed with 5 mL of MeOH:W (3:1 v/v), followed by 3 mL MeOH, and the fumonisins were eluted according to Table 1. A 4 mL portion of the eluate was dried down under nitrogen at 40 °C. The dried residue was dissolved in 500 μL MeOH, and 480 μL of 0.05 M sodium borate buffer (pH 9.5), 170 μL sodium cyanide solution (0.013%), and 50 μL of 0.5 mg/mL naphthalene-2,3 dicarboxaldehyde (NDA) in MeOH were added. The reaction mix was vortexed, heated at 60 °C for 15 min, and cooled, and 2.8 mL of 0.05 M phosphate buffer was added. FB1 and FB2 were analyzed using a Shimadzu (Kyoto, Japan) HPLC/fluorescence system (SCL10 AVP/RF-10AXL) set at 420 nm excitation and 500 nm emission, with a 10 μL injection volume. Except for PCF-I samples, all samples were analyzed in a 15 cm × 4.6 mm C18 column using a 2.5% acetic acid ACN:W gradient solvent system (55:45 v/v at time 0; 80:20 v/v after 5 min; 55:45 v/v after 8 min; 1 mL/min; 14 min run). For PCF-I samples, a 25 cm × 4.6 mm C18 column using a 3% acetic acid ACN:W system was used (45:55 v/v at time 0; 70:30 v/v after 5 min; 45:55 v/v after 18 min; 1 mL/min; 23 min run).
MATERIALS AND METHODS
Samples. A total of 208 samples (1 kg minimum per sample) of corn-based food products were collected or purchased from March 2003 to January 2005 at local retail stores in the Federal District. Products included fubá (cornmeal ) CM I), creme de milho (cornmeal ) CM II), popcorn, beiju (precooked corn flour ) PCF I), milharina (precooked corn flour ) PCF II), corn flakes, corn snacks, and sweet corn (fresh on the cob, frozen, and canned). Samples were stored in the laboratory at ambient temperature (20-30 °C), in the refrigerator (fresh corn on the cob), or in the freezer (frozen sweet corn) and were analyzed within 1 month of the collection date.
Standards and Reagent. Fumonisin (B1 and B2) and aflatoxin (B1, B2, G1, and G2) standards were purchased from Sigma-Aldrich (St. Louis, Mo). Stock solutions (1 mg/mL) of FB1 and FB2 were prepared in acetonitrile:water (1:1) and subsequently used to prepare a mixed solution containing 0.05 μg/mL of each fumonisin. Stock solutions (1 mg/mL) of each aflatoxin were prepared in benzene:acetonitrile (1:9 v/v) and used to prepare a mixed solution containing each aflatoxin at a 1 μg/mL final concentration. Acetonitrile (ACN) used was HPLC grade, and all other reagents and solvents were of analytical grade.
Fumonisin Analysis. Samples were analyzed using previously published methods (24, 25), with some modification. Samples were extracted for 30 min on a laboratory shaker using different solvent systems based on the food product being analyzed (Table 1). The extracts were filtered through qualitative Whatman filter paper, and 10 mL of the filtrate was added to a strong anion-exchange (SAX) column (Applied Separations, Allentown, PA) previously conditioned with 5 mL MeOH and 5 mL MeOH:water (1:1 v/v). The column was washed with 5 mL of MeOH:W (3:1 v/v), followed by 3 mL MeOH, and the fumonisins were eluted according to Table 1. A 4 mL portion of the eluate was dried down under nitrogen at 40 °C. The dried residue was dissolved in 500 μL MeOH, and 480 μL of 0.05 M sodium borate buffer (pH 9.5), 170 μL sodium cyanide solution (0.013%), and 50 μL of 0.5 mg/mL naphthalene-2,3 dicarboxaldehyde (NDA) in MeOH were added. The reaction mix was vortexed, heated at 60 °C for 15 min, and cooled, and 2.8 mL of 0.05 M phosphate buffer was added. FB1 and FB2 were analyzed using a Shimadzu (Kyoto, Japan) HPLC/fluorescence system (SCL10 AVP/RF-10AXL) set at 420 nm excitation and 500 nm emission, with a 10 μL injection volume. Except for PCF-I samples, all samples were analyzed in a 15 cm × 4.6 mm C18 column using a 2.5% acetic acid ACN:W gradient solvent system (55:45 v/v at time 0; 80:20 v/v after 5 min; 55:45 v/v after 8 min; 1 mL/min; 14 min run). For PCF-I samples, a 25 cm × 4.6 mm C18 column using a 3% acetic acid ACN:W system was used (45:55 v/v at time 0; 70:30 v/v after 5 min; 45:55 v/v after 18 min; 1 mL/min; 23 min run).
Fumonisins were quantified against an FB standard curve (0.005-1 ng/μL; R2 > 0.99), subjected to the same derivatization procedure as the samples. The methodology was validated for fubá (CM I), popcorn, corn snacks, corn flakes, and canned sweet corn. Control samples were fortified with known amounts of the fumonisin mixed standard dissolved in 1 mL ACN. Fortification levels (mean percent recovery) for FB1 and FB2 were 0.064 (97.3%), 0.48 (91.8%), 0.80 (79.5%), and 1.60 mg/kg (75.7%). For each matrix and level, triplicate fortified samples were analyzed; in each case, the coefficient of variation for both toxins was e20%. All samples used in the validation assays were naturally contaminated with fumonisins (0.170-0.890 mg/kg FB1 and 0.078-0.320 mg/kg FB2). Canned sweet corn contained only FB1 (0.441 mg/kg).
The limit of quantification (LOQ) was set at 0.020 mg/kg for each fumonisin, defined as the concentration of a chromatographic peak with a signal to noise ratio of 10:1. Samples with fumonisin levels below the LOQ were reported as trace levels of these toxins if the chromatographic peaks had a signal to noise ratio of at least 3:1. Each sample was analyzed in duplicate, and the mean value was reported.
Aflatoxins Analysis. Samples were analyzed according to the method described by Soares and Rodrigues-Amaya (26). Briefly, 50 g samples were extracted with 270 mL methanol and 30 mL 4% KCl and then filter-extracted and cleaned up using 150 mL 10% cupper sulfate solution and 50 g silica gel. The toxins were partitioned with 25 mL chloroform, and 5 mL of extract were evaporated to dryness, resuspended in 200 μL chloroform, and 2-10 μL were applied to a silica thin-layer chromatography (TLC) plate (Merck KGaA, Darmstadt, Germany). An AFs standard curve (1-20 ng) was also applied to the plate, which was developed in a toluene:ethyl acetate:chloroform:formic acid (35:25:25:15) system and visualized in a UV chamber. The method was successfully validated for all food matrices, with an LOQ of 2 μg/kg for each aflatoxin. This method is routinely used in the AF monitoring program in the Central Laboratory of Public Health of the Federal District (LACEN-DF), where the analyses were performed.
Food Consumption Data. Consumption data of corn-based foods were obtained from a Household Budget Survey (HBS) conducted by the Brazilian Institute of Geography and Statistics (IBGE) (27) from July 2002 to June 2003. National data were collected from 48 470 households covering the urban and rural areas of all 27 Brazilian states and the Federal District. Information on the amount of food entering the household was recorded in a diary over 7 consecutive days.
Characteristics of each household member, including age and weight (for individuals older than 20 years), were obtained through questionnaires. The weights of individuals less than 20 years in age were estimated using the U.S. National Health and Nutrition Examination Survey (28). For the underlying study, the amount of food acquired was considered as food consumed. For each household, the total weekly consumption of each food product was divided by 7 days and then by household size to generate daily consumption per individual. SAS 8.2 for Windows software (SAS Institute Inc., Cary, NC) was used to perform descriptive and statistical analysis, which included the sampling weight for each household, provided by the IBGE, to account for the whole population. To calculate the mean consumption and mean body weight for the total population, data from all households in the survey were included; for the consumers-only population, data only from households reporting consumption of corn-based products were included.
Chronic Dietary Exposure. The intake of fumonisins (FB1 + FB2) through the consumption of corn-based food in the Federal District was assessed by multiplying the mean residue levels found for the commodities analyzed in this study, by the consumption data in the Federal District estimated from the 2002/2003 HBS. At the national level, the intake was calculated using the mean levels found in this and in similar published studies conducted in Brazil, and the mean national consumption data was from the 2002/2003 HBS. The mean total FBs concentration at the national level was estimated from the mean concentration reported for each study (the present and the published ones) and the number of samples analyzed to obtain a weighted mean.
RESULTS AND DISCUSSION
Mycotoxins in Corn Products. Levels of FB1 and FB2 found in the 208 corn-based products analyzed are shown in Table 2. About 81% of the samples had FB1 levels gLOQ, and 71.6% had FB2 levels gLOQ. Eight samples had traces of FB1 and/or FB2. The FB1 and FB2 levels in the 149 samples at gLOQ were well correlated for most matrixes (R2 ) 0.55-0.92), with the exception of PCF I (R2 ) 0.22), which had a sample with ratio FB1/FB2 of 21, while for all the other samples this ratio ranged from 0.77-7 (mean of 2.3 ( 1.1).
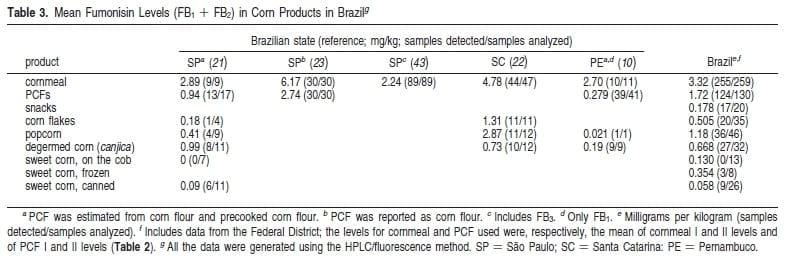
All cornmeal samples contained FB1 and FB2 at levels gLOQ. The highest concentrations of FB1 detected was 4.74 mg/kg (CM I, fubá), and the highest mean of total fumonisins was 2.04 mg/ kg (CM II, creme de milho). These products are obtained after grain degermination and milling, with creme de milho a lower size grit. This characteristic probably explains the higher FB means found in CM II, as fumonisin levels increase as grit size decreases (1). The levels of FBs found in fubá were lower than levels found in this product in other regions of the country in previous studies (Table 3). This may be due in part to lower levels of disease in the years in which the samples were collected, as well as natural variability in toxin levels found in corn from season to season (11, 12).
Milharina and beiju samples (PCF I and II) were also contaminated with fumonisins. The mean of total FBs in PCFs I and II were 0.654 and 1.09 mg/kg, respectively (Table 2). These products, referred to in some Brazilian studies as corn flour, are obtained after wet milling (PCF I) or dry milling (PCF II) of the degermed corn grain followed by a heating process.
PCF I is further submitted to extrusion, a process that can reduce fumonisin content up to 70% (13). About 62% of the corn flake and corn snack samples, which are also submitted to heating during processing, had levels of FB1 gLOQ. The highest levels, up to 0.78 mg/kg FB1, had a much lower range of fumonisin concentrations compared with all the products analyzed in this study. Machinski and Soares (21) also found low levels of fumonisin contamination in PCF and corn flake samples collected in Campinas (SP). All corn flake samples collected in the southern state of Santa Catarina (22), however, were contaminated with FB1, with a mean level of FB1 + FB2 10 times higher than those found in the present study (Table 3).
A higher incidence of samples positive for FBs, but lower means, were found in corn flake and snack samples in other countries (16, 29).
The lower contamination levels of FBs detected in PCFs, snack, and corn flake samples found in most studies can be explained by the bound fumonisins formed as a result of the heat to which these products are subjected and which cannot be detected by the method used in this study (30, 31). In this process, the fumonisins are bound to protein, starch and/or polysaccharides via the two tricarballylic acid side chains (30). Kim et al. (32) found an average of 2.6 times more FB1 present in bound form in corn flakes compared to conventional analysis. In addition to bound residues, dilution factors due to the presence of other ingredients in corn flakes and snack products are responsible for the lower levels of FBs found in these products.
About 92% of the popcorn samples analyzed were contaminated with FB1, at levels up to 1.24 mg/kg (Table 2). Popcorn grains are a type of flint or hard corn, which makes comparison with products processed from dent (soft) field corn more difficult. Studies conducted in other regions of Brazil show variability in the incidence of positive popcorn samples, as well as mean levels of total FBs (Table 3). In a study conducted in Japan (9), all 15 popcorn samples analyzed were contaminated with FBs, but at much lower levels than those found in the present study, with mean and highest FB1 levels of 0.06 and 0.35 mg/kg, respectively.
Frozen, canned, or fresh corn on the cob sweet corn samples had the lowest incidence (<21.7%) of samples positive for FB1, but the maximum concentration (1.44 mg/kg) was similar to the levels detected in popcorn and PCF samples. In Brazil, sweet corn can either be immature (milk stage) field corn or the immature stage of a high sugar content variety. Fumonisins were below the LOQ, or at low levels in canned or frozen sweet corn samples collected in Brazil (Table 3) and other countries (9, 16). These low contamination levels are expected in immature corn as fumonisin levels increase with maturation and as a consequence of higher F. Verticillioides incidence during late maturity (33, 34).
Most of the corn products evaluated in the present study originated from the southern and southeastern regions of Brazil, which are the main corn-producing regions in the country. The incidence of fumonisin contamination in corn grain grown in these regions can reach 100% (11, 35) with concentration levels varying between growing seasons, planting locations, and cultivars (33). Regional differences affect fumonisin levels within the same hybrid, regardless of Fusarium count and moisture content, suggesting an environmental influence in addition to the local predominance of toxigenic strains of the Fusarium biotype (36). Indeed, the levels of FBs found in many locations in Brazil from different growing seasons can vary widely. Vargas et al. (12) reported FB1 in 214 corn samples harvested in 1997-1998 in the central, south, and southeast regions of the country, finding 91% of the samples positive levels ranging from 0.2 to 6.1 mg/kg. All 134 samples from 48 different cultivars grown in 1994-1995 and 1997-1998 in the state of São Paulo were contaminated, with levels ranging from 0.29 to 43.8 mg/kg FB1 and 0.05 to 11.6 mg/kg FB2 (37). The FB levels found in corn grown in other countries also vary greatly (38).
Only recently have the levels of fumonisin in food and feed been under governmental regulation. Guideline levels in the United States (FB1 + FB2 + FB3) include 2 mg/kg for degermed, dry-milled corn products (<2.5% fat content) and 3 mg/kg for popcorn grain (39). In the European Community, the maximum limit (FB1 + FB2) is 1 mg/kg for corn flour, grits, semolina, germ, and oil; 0.4 mg/kg for corn-based products ready for consumption; and 0.2 mg/kg for corn-based products for babies and children (40). Brazil does not currently regulate the levels of fumonisins in food or feed. If the EC regulatory levels were to be applied in Brazil, 74% of the cornmeal samples and 29% of the samples for the products ready for consumption (PCF I, corn flakes, and snack foods) analyzed in the present study would exceed these limits.
No aflatoxins were detected in any of the 101 samples analyzed (43 fubá (CM I), 5 creme de milho (CM II), 16 beiju (PCF I), 7 milharina (PCF II), 20 popcorn, 8 corn flakes, and 2 snack food samples). Low incidence of aflatoxins in cornbased products were also found in other studies conducted in Brazil (10, 23, 41) and other countries (9). In Brazil, the aflatoxin incidence in unprocessed corn grain normally ranges from 11 to 60% of samples testing positive, with reported AFB1 levels as high as 960 μg/kg (12, 36). In general, all aflatoxincontaminated corn samples are also contaminated with fumonisins (12, 36).
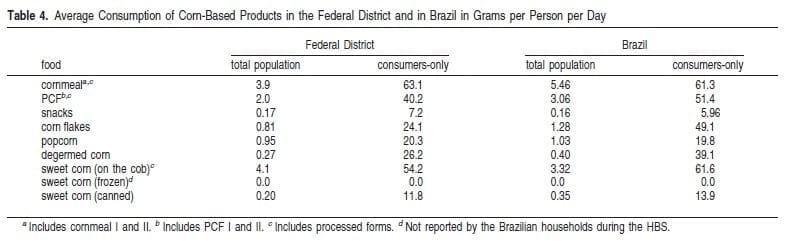
Dietary Exposure to Fumonisins from the Consumption of Corn-Based Products. Data from the 2002/2003 Brazilian HBS have shown that about 26% of the Federal District and 33.4% of the Brazilian participating households reported the acquisition of at least one corn-based product during the survey period, with cornmeal being the product most frequently acquired (by 5.5% of the households in the Federal District and by 8.5% of the Brazilian households). Mean consumption data of corn products in the Federal District and Brazil for the total population and for the consumers-only population are shown in Table 4. As no household reported the acquisition of frozen sweet corn, the consumption of this product in the country was considered to be equal to 0. On average, the estimated mean consumption of corn products in Brazil was about 25% higher than in the Federal District. The highest estimated means at both national and Federal District levels were for cornmeal, PCFs, and sweet corn on the cob.
Although HBS data reflect the food that entered the household and not the food actually consumed by its members, these data are currently the best food consumption estimates in the country and have been previously used in dietary exposure assessment studies (42). Among the limitations of HBS data that might have underestimated the values reported in Table 4 is the lack of information on the amount of food obtained outside the house. In addition, Brazilian households that did not report any food acquisition during the reporting period were not necessarily households with nonavailability/consumption of that food during a longer period. Overestimation from the HBS data may arrive from the assumption that all the food acquired during the week will be consumed by the household members during that period.
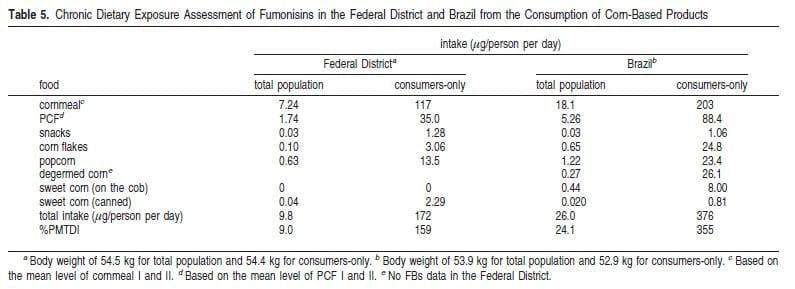
The intake of fumonisins (FB1 + FB2) from the consumption of corn-based products in the Federal District and Brazil is shown in Table 5. In both the Federal District and Brazil, cornmeal contributed the most to the intake (54-74%), followed by the PCFs (18-24%). The Brazilian total intake was over 2 times higher than intake values for the Federal District, mainly due to the high mean contamination level estimated for cornmeal and PCFs at the national level (Table 3). The risk from exposure to fumonisins from the consumption of corn products was assessed by comparing the total intake with the provisional maximum tolerable daily intake (PMTDI) of 2 μg/kg body weight per day (Table 5). This PMTDI was allocated by the JECFA to FB1, FB2, and FB3 alone or in combination, on the basis of a nonobservable-effect level of 0.2 mg/kg body weight per day from short-term and long-term renal toxicity studies in rodents and a safety factor of 100 (20). In the Federal District, the intake represented 9.0% of the PMTDI for the total population and 159% PMTDI for consumers-only. At the national level, the intake could reach 355% of the PMTDI for the consumers-only population. It should be pointed out, however, that while 2 μg/kg body weight per day is a safe dose level for regulation, a daily intake that exceeds this level may still have no observable effects.
The JECFA has also conducted an international intake estimates for FB1 using the Regional GEMS/Food diets (20). In its assessment, it was assumed that all corn and corn products consumed contained FB1 at a concentration of 1.4 mg/kg, which represented the mean level found in unprocessed corn derived from international data. The intake of FB1 and FB2 (at levels 30% of FB1) ranged from 0.91 μg/kg body weight per day in Europe to 3.1 μg/kg body weight per day in Africa. For the Latin American diet, the intake was 1.3 μg/kg body weight per day or 65% PMTDI. The Regional GEMS/Food diets were compiled from FAO Food Balance Sheet data and reflect the country production plus imports minus exports, feed, seed, industrial uses, and waste before retail. These diets are considered an overestimation of the amount of food actually consumed, mainly due to waste occurring between the retail and the consumption.
The JECFA noted in its evaluation that subsistence farmers, who grow and eat their own corn may be more exposed to fumonisins due to a higher consumption level (20). Indeed, the data from the 2002/2003 HBS showed that the total mean consumption of the corn products shown in Table 4 was over 2 times higher in rural areas than in urban areas of Brazil. For cornmeal, the mean consumption values were 4.3 and 10.7 g/person per day in urban and rural areas, respectively. The level for the urban population agrees well with the data generated by the last national consumption survey conducted by IBGE in 1977 (from 2 to 12 g/person per day) but is lower for the rural population (11-39 g/person per day). The relative decrease in consumption of cornmeal by the Brazilian rural population in the last three decades is probably due to the introduction of additional products in the diet of this population, facilitated by easer access to urban centers.
One limitation of the HBS data used in the present study is the lack of information on individual consumption patterns within the household, which prevents an exposure assessment for specific age groups of the population, e.g. children. Cornmeal is an important component of the infant diet in some Brazilian regions, normally introduced at an early age (6-12 months), and a previous assessment has shown that this population might be exposed to unsafe levels of fumonisins through the consumption of this food (43). The intake of fumonisins from cornmeal consumption by children age 6-24 months was calculated assuming a daily consumption of 20 g, estimated from nutritional recommendations and dietary patterns in the country, and the contamination level reported for Brazil in Table 3. The intake was 66.4 g/person per day, representing 313% PMDI for children (mean body weight of 10.6 kg (28)), confirming that this population may be at risk when consuming cornmeal contaminated with fumonisins at the levels found in the country.
Chronic dietary exposure to fumonisins has also been estimated in countries where the consumption of corn products is less common than in Brazil (16, 17). In The Netherlands, 37% of the population group considered to be at risk (people with gluten intolerance or with Duhring´s disease) was estimated to be exposed to an intake of at least 100 μg FB1/person per day and 97% were exposed to an intake of at least 1 μg FB1/ person per day (17). A study conducted in the United States (19) concluded that no human risk of renal toxicity would be expected at the corn contamination levels and consumption patterns for the consumers-only population in the United States. They also suggested that reducing corn consumption would have a greater impact in lowering human risk to kidney damage than lowering the level of FB permitted in corn by a similar factor.
The findings in this study support previous studies conducted in Brazil and in other countries that fumonisins are prevalent in corn products derived primarily from dried field corn. Results of the exposure assessment conducted at the local and national level have shown that certain members of the population, such as those consuming large amounts of corn products and children, might be at greater risk when consuming corn products in Brazil. Furthermore, it is essential that the Brazilian government regulate the levels of fumonisin contamination in corn and corn products and take preventive measures to enforce those levels nationally. In addition, it is important to implement in the country analytical methodologies to detect bound fumonisins in corn products submitted to heating processes to allow a more accurate exposure assessment to fumonisins by the Brazilian population.
ACKNOWLEDGMENT
We thank Saulo Cardoso Silva and João Nascimento for the aflatoxin analyses and the LACEN-DF for allowing the use of the HPLC facility. We also thank Colleen Y. Warfield for the revision of the English language.
LITERATURE CITED
(1) Katta, S. K.; Cagampang, A.E.; Jackson, L.S.; Bullerman, L.B. Distribution of Fusarium molds and fumonisins in dry-milled corn fractions. Cereal Chem. 1997, 74, 858-863.
(2) Rheeder, J. P.; Marasas, W. F. O.; Vismer, H. F. Production of Fumonisin analogs by Fusarium species. Appl. EnViron. Microbiol. 2002, 68, 2101-2105.
(3) Logrieco, A.; Mulè, G.; Moretti, A.; Bottalico, A. Toxogenic Fusarium species and mycotoxins associated with maize ear rot in Europe. Eur. J. Plant Pathol. 2002, 108, 597-609.
(4) Sydenham, E. W.; Thiel, P. G.; Marasas, W. F. O.; Shephard, G. S.; Schalkwyk, D. J.; Koch, K. R. Natural occurrence of some Fusarium mycotoxins in corn from low and high esophageal cancer prevalence areas of Transkei, Southen Africa. J. Agric. Food Chem. 1990, 38, 1900-1903.
(5) Yoshizawa, T.; Yamashita, A.; Luo, Y. Fumonisin occurrence in corn from high- and low-risk areas for human esophageal cancer in China. Appl. EnViron. Microbiol. 1994, 60, 1626-1629.
(6) Missmer, S. A.; Suarez, L.; Felkner, M.; Wang, E.; Merrill, A. H., Jr.; Rothman, K. J.; Hendricks, K. A. Exposure to fumonisins and the occurrence of neural tube defects along the Texas-Mexico border. EnViron. Health Perspect. 2006, 114, 237-241.
(7) International Agency for Research on Cancer (IARC). IARC monographs; 2006; Vols. 1-95; available at http://monographs. iarc.fr/ENG/Classification/ListagentsCASnos.pdf.
(8) Rodrigues-Amaya, D. B.; Sabino, M. Mycotoxin research in Brazil: the last decade in review. Braz. J. Microbiol. 2002, 33, 1-11.
(9) Sugita-Konishi, Y.; Nakajima, M.; Tabata, S.; Ishikuro, E.; Tanaka, T.; Norizuki, H.; Itoh, Y.; Aoyama, K.; Fujita, K.; Kai, S.; Kumagai, S. Occurrence of aflatoxins, ochratoxin A, and fumonisins in retail foods in Japan. J. Food Prot. 2006, 69, 1365-1370.
(10) Kawashima, L. M.; Soares, L. M. V. Occurrence of fumonisin B1, aflatoxins B1, B2, G1, and G2, ochratoxin A and zearalenone in corn products. Cienc. Tecnol. Aliment. 2006, 26, 516-521
(2) Rheeder, J. P.; Marasas, W. F. O.; Vismer, H. F. Production of Fumonisin analogs by Fusarium species. Appl. EnViron. Microbiol. 2002, 68, 2101-2105.
(3) Logrieco, A.; Mulè, G.; Moretti, A.; Bottalico, A. Toxogenic Fusarium species and mycotoxins associated with maize ear rot in Europe. Eur. J. Plant Pathol. 2002, 108, 597-609.
(4) Sydenham, E. W.; Thiel, P. G.; Marasas, W. F. O.; Shephard, G. S.; Schalkwyk, D. J.; Koch, K. R. Natural occurrence of some Fusarium mycotoxins in corn from low and high esophageal cancer prevalence areas of Transkei, Southen Africa. J. Agric. Food Chem. 1990, 38, 1900-1903.
(5) Yoshizawa, T.; Yamashita, A.; Luo, Y. Fumonisin occurrence in corn from high- and low-risk areas for human esophageal cancer in China. Appl. EnViron. Microbiol. 1994, 60, 1626-1629.
(6) Missmer, S. A.; Suarez, L.; Felkner, M.; Wang, E.; Merrill, A. H., Jr.; Rothman, K. J.; Hendricks, K. A. Exposure to fumonisins and the occurrence of neural tube defects along the Texas-Mexico border. EnViron. Health Perspect. 2006, 114, 237-241.
(7) International Agency for Research on Cancer (IARC). IARC monographs; 2006; Vols. 1-95; available at http://monographs. iarc.fr/ENG/Classification/ListagentsCASnos.pdf.
(8) Rodrigues-Amaya, D. B.; Sabino, M. Mycotoxin research in Brazil: the last decade in review. Braz. J. Microbiol. 2002, 33, 1-11.
(9) Sugita-Konishi, Y.; Nakajima, M.; Tabata, S.; Ishikuro, E.; Tanaka, T.; Norizuki, H.; Itoh, Y.; Aoyama, K.; Fujita, K.; Kai, S.; Kumagai, S. Occurrence of aflatoxins, ochratoxin A, and fumonisins in retail foods in Japan. J. Food Prot. 2006, 69, 1365-1370.
(10) Kawashima, L. M.; Soares, L. M. V. Occurrence of fumonisin B1, aflatoxins B1, B2, G1, and G2, ochratoxin A and zearalenone in corn products. Cienc. Tecnol. Aliment. 2006, 26, 516-521
(11) Ono, E. Y.; Sugiura, Y.; Homechin, M.; Kamogae, M.; Vizzoni, E.; Ueno, Y.; Hirooka, E. Y. Effect of climatic conditions on natural mycroflora in fumonisins freshly harvested corn of the state of Paraná, Brazil. Mycopathologia 1999, 147, 139-148.
(12) Vargas, E. A.; Preis, R. A.; Castro, L.; Silva, C. M. G. Co-occurrence of aflatoxins B1, B2, G1, G2, zearalenone and fumonisin B1 in Brazilian corn. Food Addit. Contam. 2001, 18, 981-986.
(13) Saunders, D. S.; Meredith, F. I.; Voss, K. A. Control of fumonisin:effects of processing. EnViron. Health Perspect. 2001, 109, 333- 336.
(14) Jackson, L. S.; Katta, S. K.; Fingerhut, D. D.; DeVries, J. W.; Bullerman, L. B. Effects of baking and frying on the fumonisin B1 content of corn-based foods. J. Agric. Food Chem. 1997, 45, 4800-4805.
(15) Humpf, H. U.; Voss, K. A. Effects of thermal food processing on the chemical structure and toxicity of fumonisin mycotoxins. Mol. Nutr. Food Res. 2004, 48, 255-269.
(16) Petersen, A.; Thorup, I. Preliminary evaluation of fumonisins by the Nordic countries and occurrence of fumonisins (FB1 and FB2) in corn-based food on the Danish market. Food Addit. Contam. 2001, 18, 221-226.
(17) Kuiper-Goodman, T.; Scott, P. M.; McEwen, N. P.; Lombaert, G. A.; Ng, W. Approaches to the risk assessment of fumonisins in corn-based foods in Canada. AdV. Exp. Med. Biol. 1996, 392, 369-393.
(18) de Nijs, M.; van Egmond, H. P.; Nauta, M.; Rombouts, F. M.; Notermans, S. H. Assessment of human exposure to fumonisin B1. J. Food Prot. 1998, 61, 879-884.
(19) Humpreys, S. H.; Carrrington, C.; Bolger, M. A quantitative risk assessment for fumonisins B1 and B2 in US corn. Food Addit. Contam. 2001, 18, 311-220.
(20) Joint FAO/WHO Expert Committee on Food Additives. EValuation of Certain Mycotoxins in Food; WHO Food Additive Series 47; WHO: Geneva, Switzerland, 2001; available at http://www.inchem.org/documents/jecfa/jecmono/v47je03.htm.
(21) Machinski, M.; Soares, L. M. V. Fumonisins B1 and B2 in Brazilian corn-based food products. Food Addit. Contam. 2000, 17, 875-879.
(22) Scaff, R. M. C.; Scussel, V. M. Fumonisins B1 and B2 in corn-based products commercialized in the state of Santa Catarina-southern Brazil. Braz. Arch. Biol. Technol. 2004, 47, 911-919.
(23) Bittencourt, A. B. F.; Oliveira, C. A.V.; Dilkin, P.; Corrêa, B. Mycotoxin occurrence in cornmeal and flour traded in São Paulo, Brazil. Food Control 2005, 16, 117-120.
(24) Sydenham, E. W.; Shephard, G. S.; Thiel, P. G.; Stockenstrom, S.; Snijman, P. W.; Van Schalkwyk, D. J. Liquid chromatographic determination of Fumonisins B1, B2 and B3 in corn: AOAC -IUPAC collaborative study. J. AOAC Int. 1996, 79, 688-696.
(25) Caldas, E. D.; Sadilkova, K.; Ward, B.; Jones, A. D.; Winter, C. K.; Gilchrist, D. G. Biosynthetic Studies of Fumonisin B1 and AAL Toxins. J. Agric. Food Chem. 1998, 46, 4734-4743.
(26) Soares, L. M. V.; Rodrigues-Amaya, D. B. Survey of aflatoxins, ochratoxin A, zearalenone and sterigmatocystin in some Brazilian foods by using a multi-toxin thin layer chromatography method. J. AOAC Int. 1989, 72, 22-26.
(27) Instituto Brasileiro de Geografia e Estatística. IBGE Pesquisa de orçamentos familiares 2002-2003. Raw data; IBGE: Rio de Janeiro, Brazil, January 18, 2005.
(28) National Center For Health Statistics. CDC Growth Charts: United States. Weight-for-age charts, 2 to 20 years, LMS parameters and selected smoothed weight percentiles in kilograms, by sex and age. http://www.cdc.gov/nchs/about/major/nhanes/growthcharts/ datafiles.htm (accessed 2006).
(29) Solovey, M. M. S.; Somoza, C.; Cano, G.; Pacin, A.; Resnik, S. A survey of fumonisins, deoxynivalenol, zearalenone and aflatoxins contamination in corn-based food products in Argentina. Food Addit. Contam. 1999, 16, 325-329.
(30) Seefelder, W.; Knecht, A.; Humpf, H. U. Bound fumonisin B1: analysis of fumonisin-B1 glyco and amino acid conjugates byliquid chromatography-electrospray ionization-tandem mass spectrometry. J. Agric. Food Chem. 2003, 51, 5567-73.
(31) Lu, Y.; Clifford, L.; Hauck, C. C.; Hendrich, S.; Osweiler, G.; Murphy, P. A. Characterization of fumonisin B1- glucose reactionkinetics and products. J. Agric. Food Chem. 2002, 50, 4726-4733.
(32) Kim, E. K.; Scott, P. M.; Lau, B. P. Hidden fumonisin in corn flakes. Food Addit. Contam. 2003, 20, 161-169.
(33) Almeida, A. P.; Fonseca, H.; Fancelli, A. L.; Direito, G. M.; Ortega, E. M.; Correa, B. Mycoflora and fumonisin contamination in Brazilian corn from sowing to harvest. J. Agric. Food Chem. 2002, 50 (13), 3877-3882.
(34) Warfield, C. Y.; Gilchrist, D. G. Influence of kernel age on fumonisin B1 production in maize by Fusarium moniliforme. Appl. EnViron. Microbiol. 1999, 65, 2853-2856.
(35) van der Westhuizen, L.; Shephard, G. S.; Scussel, V. M.; Costa, L. L.; Vismer, H. F.; Rheeder, J. P.; Marasas, W. F. Fumonisin contamination and Fusarium incidence in corn from Santa Catarina, Brazil. J. Agric. Food Chem. 2003, 51, 5574-5578.
(36) Ono, E. Y.; Ono, M. A.; Funo, F. Y.; Medinal, A. E.; Oliveira, T. C.; Kawamura, O.; Ueno, Y.; Hirooka, E. Y. Evaluation of fumonisin-aflatoxin co-occurrence in Brazilian corn hybrids by ELISA. Food Addit. Contam. 2001, 18, 719-729.
(37) Camargos, S. M.; Soares, L. M.; Sawazaki, E.; Bolonhezi, D.; Castro, J. L.; Bortolleto, N. Fumonisins in corn cultivars in the state of São Paulo. Braz. J. Microbiol. 2000, 31, 226-229.
(38) Shephard, G. S.; Marasas, W. F. O.; Leggott, N. L.; Yasdanpanah, H.; Rahimian, H.; Safavi, N. Natural Occurrence of Fumonisins in Corn from Iran. J. Agric. Food Chem. 2000, 48, 1860- 1864.
(39) Fumonisin Levels in Human Foods and Animal Feeds. Guidance for Industry, Final guidance; US Food and Drug Administration, Center for Food Safety and Applied Nutrition; Rockville, MD, November, 2001; available at http://www.cfsan.fda.gov/∼dms/ fumongu2.html.
(40) Commission Regulation (EC) Setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Commission 2006, L364, 5-24.
(41) Silva, S. C.; Oliveira, J. N.; Caldas, E. D. Aflatoxins in food commercialized in the Federal District from 1985 to 1995. ReV. Inst. Adolfo Lutz 1996, 56, 49-52.
(42) Caldas, E. D.; Tressou, J.; Boon, P. Dietary exposure of Brazilian consumers to dithiocarbamate pesticides - a probabilistic approach. Food Chem. Toxicol. 2006, 44, 1562-1571.
(43) Castro, M. F.; Shephard, G. S.; Sewram, V.; Vicente, E.; Mendonça, T. A.; Jordan, A. C. Fumonisins in Brazilian cornbased foods for infant consumption. Food Addit. Contam. 2004, 21, 693-699.
Received for review May 2, 2007. Revised manuscript received July 11, 2007. Accepted July 15, 2007.
(38) Shephard, G. S.; Marasas, W. F. O.; Leggott, N. L.; Yasdanpanah, H.; Rahimian, H.; Safavi, N. Natural Occurrence of Fumonisins in Corn from Iran. J. Agric. Food Chem. 2000, 48, 1860- 1864.
(39) Fumonisin Levels in Human Foods and Animal Feeds. Guidance for Industry, Final guidance; US Food and Drug Administration, Center for Food Safety and Applied Nutrition; Rockville, MD, November, 2001; available at http://www.cfsan.fda.gov/∼dms/ fumongu2.html.
(40) Commission Regulation (EC) Setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Commission 2006, L364, 5-24.
(41) Silva, S. C.; Oliveira, J. N.; Caldas, E. D. Aflatoxins in food commercialized in the Federal District from 1985 to 1995. ReV. Inst. Adolfo Lutz 1996, 56, 49-52.
(42) Caldas, E. D.; Tressou, J.; Boon, P. Dietary exposure of Brazilian consumers to dithiocarbamate pesticides - a probabilistic approach. Food Chem. Toxicol. 2006, 44, 1562-1571.
(43) Castro, M. F.; Shephard, G. S.; Sewram, V.; Vicente, E.; Mendonça, T. A.; Jordan, A. C. Fumonisins in Brazilian cornbased foods for infant consumption. Food Addit. Contam. 2004, 21, 693-699.
Received for review May 2, 2007. Revised manuscript received July 11, 2007. Accepted July 15, 2007.
Related topics:
Recommend
Comment
Share
9 de noviembre de 2011
This is an excellent accomplishment by Caldas and Silva from University of Brasilia in the field of mycotoxins in corn based food products consumed in Brazil. More of such studies are needed to be done especially in the developing nations. However, the obstacles are how to get fumonisins kits and the inavailability of HPLC in most laboratories in the 3rd world.
Prof. Afolabi Oluwadun,
Dept. Medical Microbiology
College of Health Sciences
Olabisi Onabanjo University
P.O.Box657
Sagamu, Ogun State, NIGERIA. Tel.+2348033871945
NIGERIA
Recommend
Reply

Would you like to discuss another topic? Create a new post to engage with experts in the community.