Mycotoxins in major commercial food crops
Fungal mycotoxins in food commodities: present status and future concerns
Author details:
Mycotoxins are toxic secondary metabolites produced by fungi when they colonies the foodstuffs. These are potent toxins having severe health consequences in people, being mutagenic, teratogenic, and carcinogenic. In agricultural commodities, the contamination of mycotoxins is more prevalent. Several fungi can produce mycotoxins on agricultural products during harvest or in postharvest, and they have significant adverse effects on both animal and human beings. The most prevalent mycotoxins found in food commodities are aflatoxins and ochratoxins produced by Aspergillus species, ochratoxins and patulin produced by Penicillium, as well as fumonisins, deoxynivalenol, and zearalenone produced by Fusarium species. Worldwide, fumonisins, patulin, aflatoxins, and ochratoxins, among others, are responsible for numerous acute and chronic diseases in people and domestic animals. In food commodities, mycotoxins have been quantified and detected using various analytical methods. Besides, mycotoxins occurrence in food commodities were decontaminated through many potential approaches, such as physical, chemical, and biological methods. This review summarizes the findings of 30 years of research into mycotoxins in major commercial food crops including wheat, maize, sorghum, pearl millet, peanut, oat, pulses, barley, oilseeds, rice, and fruits and fruit juices. We also discuss the detection methods of major mycotoxins, available decontamination strategies along with their disadvantages and knowledge gaps. It is anticipated that data from meticulous studies on mycotoxins in food commodities will help in the development of safer food and in setting priorities for future research.
KEYWORDS ochratoxin, food commodities, aflatoxins, fumonisins, physical methods, essential oils
Introduction
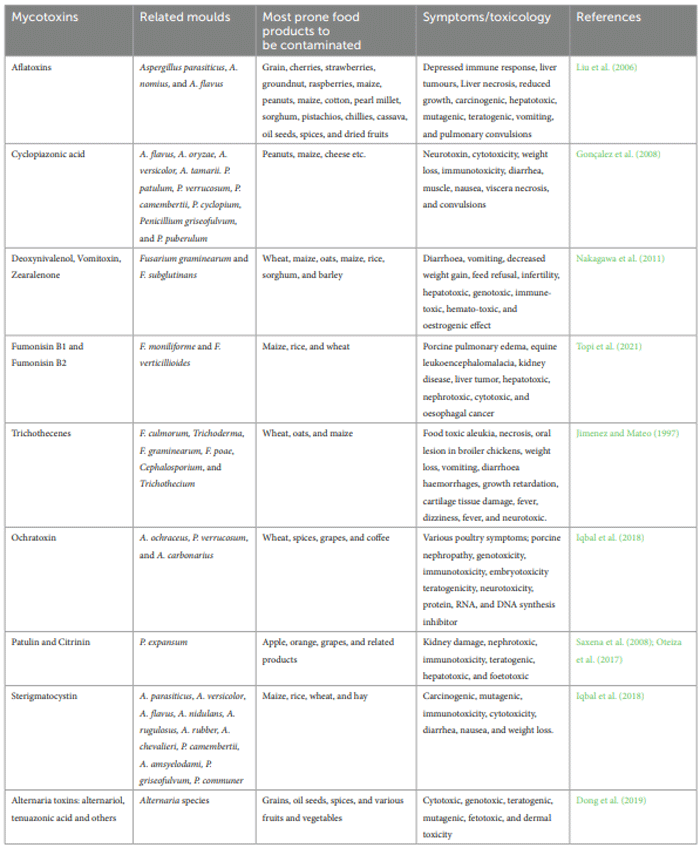
Classification of foodborne mycotoxins
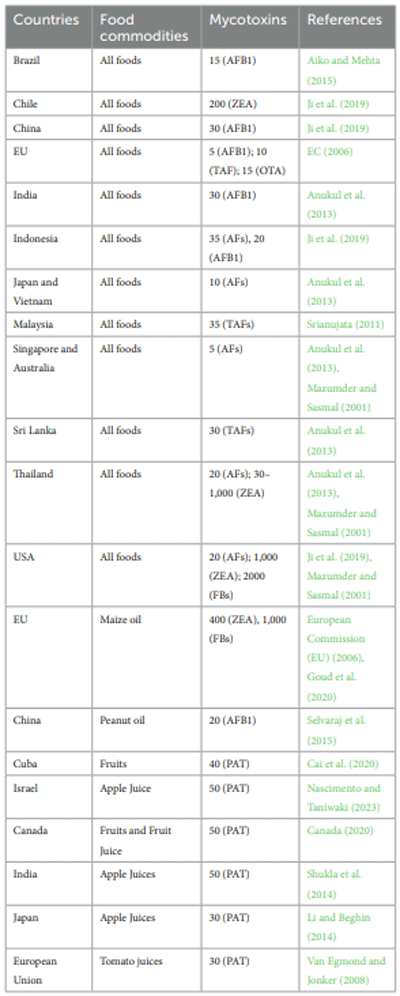
Mycotoxin regulations in food commodities
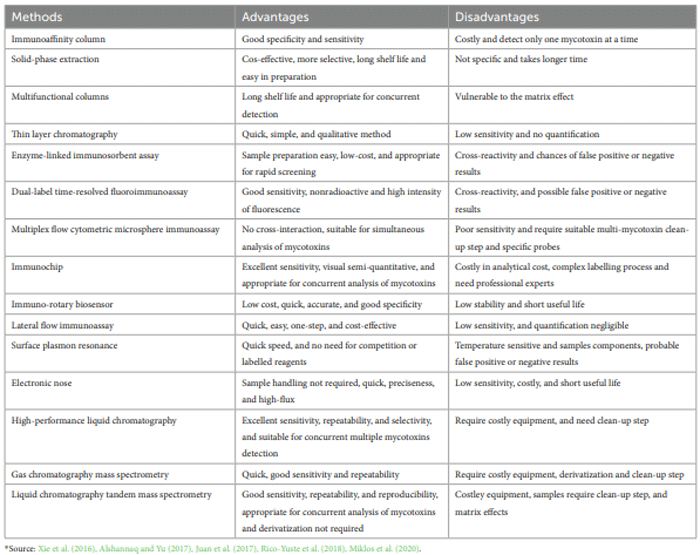
Methods of detecting mycotoxins
Extraction and purification of mycotoxins
Detection of mycotoxins
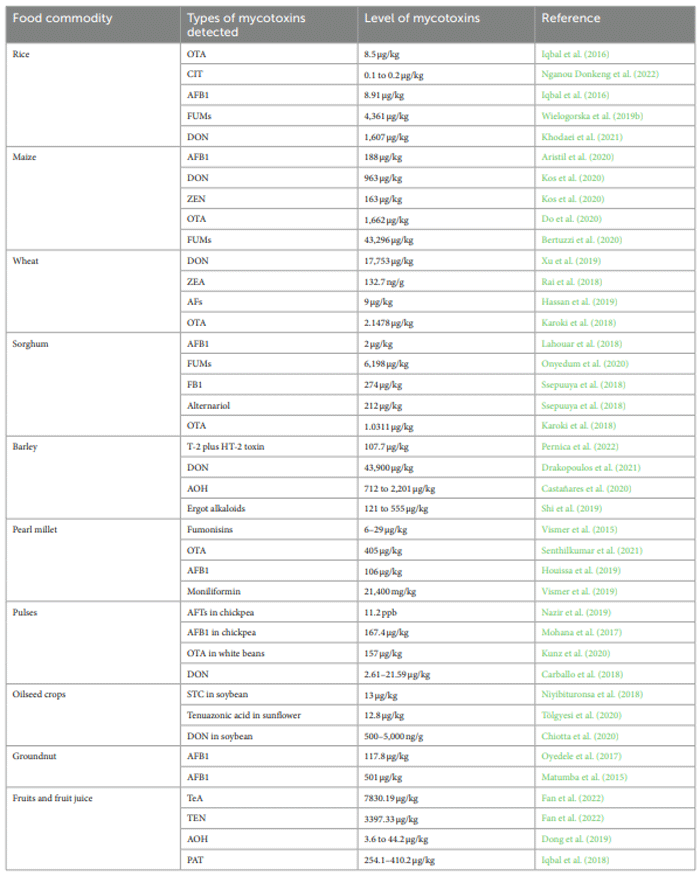
Occurrence of mycotoxins in food commodities
Rice (Oryza sativa L.)
Maize (Zea mays L.)
Wheat (Triticum aesticum L.)
Other small cereal food crops
Pulses and oilseeds crops
Peanut (Arachis hypogaea L.)
Fruits and fruit juices
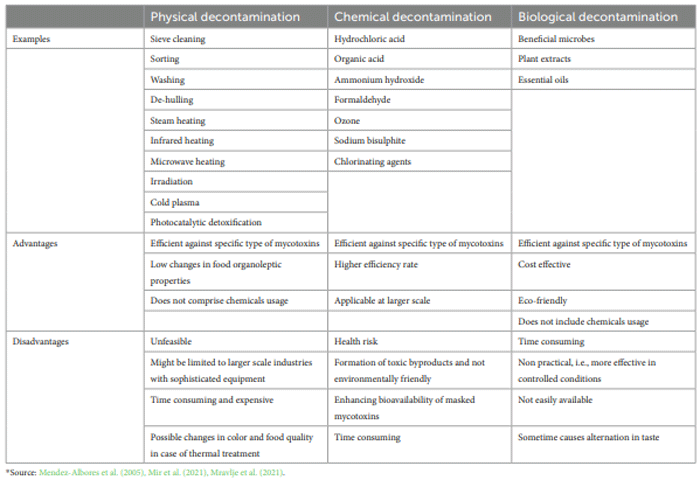
Decontamination strategies of mycotoxins in food commodities
Chemical methods of decontamination of mycotoxins
FIGURE 2 Ideal characteristics of mycotoxins’ detoxification processes applied to food commodities.

Physical approaches of decontamination of mycotoxins
Irradiation
Pulsed light treatment
Cold plasma
Biological methods of detoxification of mycotoxins
Use of beneficial microbes
Use of botanicals
EOs for detoxification of AFs
EOs for detoxification of ZEA
EOs for detoxification of FBs
EOs for detoxification of OTAs
Conclusions and perspectives
Author contributions
Conflict of interest
Publisher’s note
Abbas, H. K., Zablotowicz, R. M., and Bruns, H. A. (2008). Modelling the colonization of maize by toxigenic and non-toxigenic Aspergillus flavus strains: implications for biological control. World Mycotoxin J. 1, 333–340. doi: 10.3920/WMJ2008.x036
Abdelhamid, A. A. (1990). Occurrence of some mycotoxins (aflatoxins, ochratoxin A, citrinin, zearalenone and vomitoxin) in various Egyptian feeds. Arch. Anim. Nutr. 40, 647–664.
Abdolmaleki, K., Khedri, S., Alizadeh, L., and Javanmardi, F. (2021). The mycotoxins in edible oils: an overview of prevalence, concentration, toxicity, detection and decontamination techniques. Trends Food Sci. Technol. 115, 500–511. doi: 10.1016/j.tifs.2021.06.057
Abramson, D., Clear, R. M., Usleber, E., Gessler, R., Nowicki, T. W., and Martlbauer, E. (1998). Fusarium species and 8 keto-trichothecene mycotoxins in Manitoba barley. Cereal Chem. 75, 137–141.
Abuagela, M. O., Iqdiam, B. M., Mostafa, H., Marshall, S. M., Yagiz, Y., Marshall, M. R., et al. (2019). Combined effects of citric acid and pulsed light treatments to degrade B-aflatoxins in peanut. Food Bioprod. Process. 117, 396–403. doi: 10.1016/j. fbp.2019.08.011
Abu-Bakar, N. B., Makahleh, A., and Saad, B. (2014). Vortex-assisted liquid-liquid microextraction coupled with high performance liquid chromatography for the determination of furfurals and patulin in fruit juices. Talanta 120, 47–54. doi: 10.1016/j. talanta.2013.11.081
Adebo, O. A., Njobeh, P. B., Adebiyi, J. A., and Kayitesi, E. (2018). Coinfluence of fermentation time and temperature on physicochemical properties, bioactive components and microstructure of ting (a Southern African food) from whole grain sorghum. Food Biosci. 25, 118–127. doi: 10.1016/j.fbio.2018.08.007
Adefolalu, F. S., Apeh, D. O., Salubuyi, S. B., Galadima, M., Agbo, A. O., Makun, H. A., et al. (2021). Quantitative appraisal of total aflatoxin in ready-to-eat groundnut in northCentral Nigeria. J. Chem. Risk 12, 25–31. doi: 10.22034/JCHR.2021.1911495.1196
Adeyeye, S. A. (2016). Fungal mycotoxins in foods: A review. Cogent Food Agric. 2:1213127. doi: 10.1080/23311932.2016.1213127
Adjou, E. S., Dahouenon-Ahoussi, E., Degnon, R., Soumanou, M. M., and Sohounhloue, D. C. K. (2012). Investigations on bioactivity of essential oil of Ageratum conyzoides L., from Benin against the growth of fungi and aflatoxin production. Int. J. Pharma Sci. Rev. Res. 13, 143–148.
Afolabi, C. G., Ezekiel, C. N., Ogunbiyi, A. E., Oluwadairo, O. J., Sulyok, M., and Krska, R. (2019). Fungi and mycotoxins in cowpea (Vigna unguiculata L) on Nigerian markets. Food Addit. Contam. Part B Surv. 13, 52–58. doi: 10.1080/19393210.2019.1690590
Agriopoulou, S., Stamatelopoulou, E., and Varzakas, T. (2020). Advances in analysis and detection of major mycotoxins in foods. Foods 9:518. doi: 10.3390/foods9040518
Ahmad, M. S., and Sinha, K. K. (2002). Mycotoxin contamination of mustard seeds during storage. Indian Phytopath. 55, 299–302.
Aiko, V., and Mehta, A. (2015). Occurrence, detection and detoxification of mycotoxins. J. Biosci. 40, 943–954. doi: 10.1007/s12038-015-9569-6
Aksoy, U. R., Eltem, K. B., Meyvaci, A. A., and Karabat, S. (2007). Five-year survey of Ochratoxin A in processed sultanas from Turkey. Food Addit. Contam. 24, 292–296. doi: 10.1080/02652030601039021
Al-Anati, L., and Petzinger, E. (2006). Immunotoxic activity of ochratoxin A. J. Vet. Pharmacol. Ther. 29, 79–90. doi: 10.1111/j.1365-2885.2006.00718.x
Al-Hazmi, N. (2010). Determination of patulin and ochratoxin A using HPLC in apple juice samples in Saudi Arabia. Saudi J. Biol. Sci. 17, 353–359. doi: 10.1016/j.sjbs.2010.06.006
Alpsoy, L. (2010). Inhibitory effect of essential oil on aflatoxin activities. Afr. J. Biotechnol. 17, 2474–2481.
Alshannaq, A., and Yu, J. H. (2017). Occurrence, toxicity, and analysis of major mycotoxins in food. Int. J. Environ. Res. Public Health 14:632. doi: 10.3390/ ijerph14060632
Andrade, P. D., Gomes da Silva, J. L., and Caldas, E. D. (2013). Simultaneous analysis of aflatoxins B1, B2, G1, G2, M1 and ochratoxin A in breast milk by high-performance liquid chromatography/fluorescence after liquid-liquid extraction with low temperature purification (LLE-LTP). J. Chromatogr. A 1304, 61–68. doi: 10.1016/j.chroma.2013.06.049
Ansari, F., Khodaiyan, F., Rezaei, K., and Rahmani, A. (2015). Modelling of aflatoxin G1 reduction by kefir grain using response surface methodology. J. Environ. Health Sci. Eng. 13:40. doi: 10.1186/s40201-015-0190-2
Anukul, N., Vangnai, K., and Mahakarnchanakul, W. (2013). Significance of regulation limits in mycotoxin contamination in Asia and risk management programs at the national level. J. Food Drug Anal. 21, 227–241. doi: 10.1016/j.jfda.2013.07.009
Aquino, S., Ferreira, F., Ribeiro, D. H. B., Correa, B., Greiner, R., and Villavicencio, A. L. C. H. (2005). Evaluation of viability of Aspergillus flavus and aflatoxins degradation in irradiated samples of maize. Braz. J. Microbiol. 36, 352–356. doi: 10.1590/ S1517-83822005000400009
Aristil, J., Venturini, G., Maddalena, G., Toffolatti, S. L., and Spada, A. (2020). Fungal contamination and aflatoxin content of maize, moringa and peanut foods from rural subsistence farms in South Haiti. J. Stored Prod. Res. 85:101550. doi: 10.1016/j. jspr.2019.101550
Aroyeun, S. O., Adegoke, G. O., Varga, J., and Teren, J. (2009). Reduction of aflatoxin B1 and ochratoxin A in cocoa beans infected with Aspergillus via Ergosterol value. World Rev. Sci. Technol. Sustain. Dev. 6, 75–89. doi: 10.1504/WRSTSD.2009.022459
Aydin, A., Aksu, H., and Gunsen, U. (2011). Mycotoxin levels and incidence of mould in Turkish rice. Environ. Monit. Assess. 178, 271–280. doi: 10.1007/ s10661-010-1688-9
Aziz, N. H., Attia, E. S. A., and Farag, S. A. (1997). Effect of gamma-irradiation on the natural occurrence of Fusarium mycotoxins in wheat, flour and bread. Food Nahrung 41, 34–37. doi: 10.1002/food.19970410109
Aziz, N. H., Ferial, M., Shahin, A. A., and Roushy, S. M. (2007). Control of Fusarium moulds and fumonisin B1 in seeds by gamma-irradiation. Food Control 18, 1337–1342. doi: 10.1016/j.foodcont.2005.12.013
Aziz, N. H., Moussa, L. A., and Far, F. M. (2004). Reduction of fungi and mycotoxins formation in seeds by gamma-radiation. J. Food Saf. 24, 109–127. doi: 10.1111/j.1745-4565.2004.tb00379.x
Badiale-Furlong, E., Dors, G. C., Oliveira, M., Dos, S., de Souza, M. M., and Kuhn, R. C. (2003). “Avaliacaodaqualidade de farinha de trigoeprodutos de panificaçãocomercializadas no Rio Grande do Sul” in Simpósio de Ciências de Alimentos e Saude (Anais: Florianópolis-SC/UFSC), 1–4.
Bankole, S. A. (1997). Effect of essential oils from two Nigerian medicinal plants (Azadirachta indica and Morinda lucida) on growth and aflatoxin B1 production in maize grain by a toxigenic Aspergillus flavus. Lett. Appl. Microbiol. 24, 190–192.
Basílico, M. Z., and Basílico, J. C. (1999). Inhibitory effects of some spice essential oils on aspergillus ochraceus NRRL 3174 growth and ochratoxin A production. Lett. Appl. Microbiol. 29, 238–241. doi: 10.1046/j.1365-2672.1999.00621.x
Begum, H., and Samajpati, N. (2000). Mycotoxin production on rice, pulses and oilseeds. Naturwissenschaften 87, 275–277. doi: 10.1007/s001140050720
Beretta, B., Gaiaschib, A., Galli, C. L., and Restani, P. (2000). Patulin in apple-based foods: occurrence and safety evaluation. Food Addit. Contam. 17, 399–406. doi: 10.1080/026520300404815
Berthiller, F., Cramer, B., Iha, M. H., Krska, R., Lattanzio, V. M. T., MacDonald, S., et al. (2017). Developments in mycotoxin analysis: an update for 2016-2017. World Mycotoxin J. 11, 5–32. doi: 10.3920/WMJ2017.2250
Bertuzzi, T., Giorni, P., Rastelli, S., Vaccino, P., Lanzanova, C., and Locatelli, S. (2020). Co-occurrence of moniliformin and regulated Fusarium toxins in maize and wheat grown in Italy. Molecules 25, 1–13. doi: 10.3390/molecules25102440
Bhat, R., and Reddy, K. R. N. (2017). Challenges and issues concerning mycotoxins contamination in oil seeds and their edible oils: updates from last decade. Food Chem. 215, 425–437. doi: 10.1016/j.foodchem.2016.07.161
Bhatt, R. V., Shetty, P. H., Amruth, R. P., and Sudershan, R. V. (1997). A foodborne disease outbreak due to the consumption of moldy sorghum and maize containing fumonisin mycotoxins. Clin. Toxicol. 35, 249–255.
Birck, N. M. M., Lorini, I., and Scussel, V. M. (2003). “Sanitary conditions and mycotoxins in wheat grains (Triticum aestivum) and flour (common and special) through milling processing” in IV Congreso Latino americano De Mycotoxicologia (LaHabana, Cuba: Anais)
Bottalico, A., and Perrone, G. (2002). Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur. J. Plant Pathol. 108, 611–624. doi: 10.1023/A:1020635214971
Burcu Aydın, E., Aydın, M., and Kemal Sezgintürk, M. (2020). Biosensors and the evaluation of food contaminant biosensors in terms of their performance criteria. Int. J. Environ. Anal. Chem. 100, 602–622. doi: 10.1080/03067319.2019.1672675
Cai, Y., McLaughlin, M., and Zhang, K. (2020). Advancing the FDA/office of regulatory affairs mycotoxin program: new analytical method approaches to addressing needs and challenges. J. AOAC Int. 103, 705–709. doi: 10.1093/jaocint/qsz007
Cairns, V., and Magan, N. (2003). “Impact of essential oils on growth and ochratoxin A production by Penicillium verrucosum and Aspergillus ochraceus on a wheat-based substrate.” in Advances in stored product protection. Proceedings of the 8th International Working Conference on Stored Product Protection, York, UK, 22–26 July 2002. Edited by Credland, P.F., Armitage, D.M., Bell, C.H., Cogan, P.M., Highley, E., 479–485.
Campbell, H., Choo, T. M., Vigier, B., and Underhill, L. (2000). Mycotoxins in barley and oat samples from Eastern Canada. Can. J. Plant Sci. 80, 977–980. doi: 10.4141/ P00-042
Canada, H. (2020). Health Canada’s maximum levels for chemical contaminants in foods (Ontario: Health Canada Ottawa).
Carballo, D., Font, G., Ferrer, E., and Berrada, H. (2018). Evaluation of mycotoxin residues on ready-to-eat food by chromatographic methods coupled to mass spectrometry in tandem. Toxins 10:243. doi: 10.3390/toxins10060243
Castañares, E., Pavicich, M. A., Dinolfo, M. I., Moreyra, F., Stenglein, S. A., and Patriarca, A. (2020). Natural occurrence of Alternaria mycotoxins in malting barley grains in the main producing region of Argentina. J. Sci. Food Agric. 100, 1004–1011. doi: 10.1002/jsfa.10101
Chala, A., Mohammed, A., Ayalew, A., and Skinnes, H. (2013). Natural occurrence of aflatoxins in groundnut (Arachis hypogaeaL.) from eastern Ethiopia. Food Control 30, 2602–2605. doi: 10.1016/j.foodcont.2012.08.023
Chen, D., Chen, P., Cheng, Y., Peng, P., Liu, J., Ma, Y., et al. (2019). Deoxynivalenol decontamination in raw and germinating barley treated by plasma-activated water and intense pulsed light. Food Bioprocess Technol. 12, 246–254. doi: 10.1007/ s11947-018-2206-2
Chen, X., Qiu, Y., Zhang, J., Guo, Y., Ding, Y., and Lyu, F. (2022). Degradation efficiency and products of deoxynivalenol treated by cold plasma and its application in wheat. Food Control 136:108874. doi: 10.1016/j.foodcont.2022.108874
Chiotta, M. L., Fumero, M. V., Cendoya, E., Palazzini, J. M., Alaniz-Zanon, M. S., Ramirez, M. L., et al. (2020). Toxigenic fungal species and natural occurrence of mycotoxins in crops harvested in Argentina. Rev. Argent. Microbiol. 52, 339–347. doi: 10.1016/j.ram.2020.06.002
Cho, M. S., Kim, K., Seo, E., Kassim, N., Mtenga, A. B., Shim, W. B., et al. (2010). Occurrence of patulin in various fruit juices from South Korea: an exposure assessment. Food Sci. Biotechnol. 19, 1–5. doi: 10.1007/s10068-010-0001-6
Chulze, S. N., Magnoli, C. E., and Dalcero, A. M. (2006). Occurrence of Ochratoxin A in wine and ochratoxigenic mycoflora in grape and dried vine fruits in South America. Int. J. Food Microbiol. 111, S5–S9. doi: 10.1016/j.ijfoodmicro.2006.02.006
Claeys, L., Romano, C., De Ruyck, K., Wilson, H., Fervers, B., Korenjak, M., et al. (2020). Mycotoxin exposure and human cancer risk: A systematic review of epidemiological studies. Compr. Rev. Food Sci. Food Saf. 19, 1449–1464. doi: 10.1111/1541-4337.12567
Dambolena, J. S., López, A. G., Cánepa, M. C., Theumer, M. G., Zygadlo, J. A., and Rubinstein, H. R. (2008). Inhibitory effect of cyclic terpenes (limonene, menthol, menthone and thymol) on Fusarium verticillioides MRC 826 growth and fumonisin B1 biosynthesis. Toxicon 51, 37–44. doi: 10.1016/j.toxicon.2007.07.005
Do, T. H., Tran, S. C., Le, C. D., Nguyen, H. B. T., Le, P. T. T., Le, H. H. T., et al. (2020). Dietary exposure and health risk characterization of aflatoxin B1, ochratoxin A, fumonisin B1, and zearalenone in food from different provinces in Northern Vietnam. Food Control 112:107108. doi: 10.1016/j.foodcont.2020.107108
Dong, M., Si, W., Jiang, K., Nie, D., Wu, Y., Zhao, Z., et al. (2015). Multi-walled carbon nanotubes as solid-phase extraction sorbents for simultaneous determination of type A trichothecenes in maize, wheat and rice by ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 1423, 177–182. doi: 10.1016/j.chroma.2015.10.068
Dong, H., Xian, Y., Xiao, K., Wu, Y., Zhu, L., and He, J. (2019). Development and comparison of single-step solid phase extraction and QuEChERS clean-up for the analysis of 7 mycotoxins in fruits and vegetables during storage by UHPLC-MS/MS. Food Chem. 274, 471–479. doi: 10.1016/j.foodchem.2018.09.035
Drakopoulos, D., Sulyok, M., Krska, R., Logrieco, A. F., and Vogelgsang, S. (2021). Raised concerns about the safety of barley grains and straw: A Swiss survey reveals a high diversity of mycotoxins and other fungal metabolites. Food Control 125:107919. doi: 10.1016/j.foodcont.2021.107919
EC (2006). Commission regulation (EC) no 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union.
Ediage, E. N., Di Mavungu, J. D., Monbaliu, S., Peteghem, C. V., and De Saeger, S. (2011). A validated multianalyte LC–MS/MS method for quantification of 25 mycotoxins in cassava flour, peanut cake and maize samples. J. Agric. Food Chem. 59, 5173–5180. doi: 10.1021/jf2009364
Edwards, S.G. (2007). Investigation of Fusarium mycotoxins in UK barley and oat production. Project Report No.415, AHDB Cereals & Oilseeds: Stoneleigh, UK.
El Darra, N., Gambacorta, L., and Solfrizzo, M. (2019). Multi mycotoxins occurrence in spices and herbs commercialized in Lebanon. Food Control 95, 63–70. doi: 10.1016/j. foodcont.2018.07.033
El-Nagerabi, S. A. F., Al-Bahry, S. N., Elshafie, A. E., and AlHilali, S. (2012). Effect of Hibiscus sabdariffa extract and Nigella sativa oil on the growth and aflatoxin B1 production of Aspergillus flavus and Aspergillus parasiticus strains. Food Control 25, 59–63. doi: 10.1016/j.foodcont.2011.09.033
El-Nagerabi, S. A. E., Elshafie, A. E., AlKhanjari, S. S., Al-Bahry, S. N., and Elamin, M. R. (2013). The potential of baobab (Adansonia digitata L.) extracts as biocontrol on the growth and aflatoxin production by Aspergillus flavus and A. parasiticus. J. Food Res. 2, 93–103. doi: 10.5539/jfr.v2n3p93
Embaby, E. M., Reda, M., Abdel-Wahhab, M. A., Omara, H., and Mokabel, A. M. (2013). Occurrence of toxigenic fungi and mycotoxins in some legume seeds. J. Agric. Technol. 9, 151–164.
Esper, R. H., Gonçalez, E., Marques, M. O. M., Felicio, R. C., and Felicio, J. D. (2014). Potential of essential oils for protection of grains contaminated by aflatoxin produced by Aspergillus flavus. Front. Microbiol. 5:269. doi: 10.3389/fmicb.2014.00269
European Commission (EU) (2006). European Union Commission regulation no. 1881/2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 364, 5–24.
Ezekiel, C. N., Sulyok, M., Warth, B., Odebode, A. C., and Krska, R. (2012). Natural occurrence of mycotoxins in peanut cake from Nigeria. Food Control 27, 338–342. doi: 10.1016/j.foodcont.2012.04.010
Fan, Y., Liu, F., He, W., Qin, Q., Hu, D., Wu, A., et al. (2022). Screening of multimycotoxins in fruits by ultra-performance liquid chromatography coupled to ion mobility quadrupole time-of-flight mass spectrometry. Food Chem. 368:130858. doi: 10.1016/j.foodchem.2021.130858
Farzaneh, M., Shi, Z. Q., Ghassempour, A., Sedaghat, N., Ahmadzadeh, M., Mirabolfathy, M., et al. (2012). Aflatoxin B1 degradation by Bacillus subtilis UTBSP1 isolated from pistachio nuts of Iran. Food Control 23, 100–106. doi: 10.1016/j. foodcont.2011.06.018
Fox, T., and Fimeche, C. (2013). Global food waste not, Want Not. IMechE. England and Wales.
Gandomi, H., Misaghi, A., Basti, A. A., Bokaei, S., Khosravi, A., Abbasifar, A., et al. (2009). Effect of Zataria multiflora Boiss. essential oil on growth and aflatoxin formation by Aspergillus flavus in culture media and cheese. Food Chem. Toxicol. 47, 2397–2400. doi: 10.1016/j.fct.2009.05.024
Gbashi, S., Madala, N. E., De Saeger, S., De Boevre, M., and Njobeh, P. B. (2019). Numerical optimization of temperature-time degradation of multiple mycotoxins. Food Chem. Toxicol. 125, 289–304. doi: 10.1016/j.fct.2019.01.009
Gemeda, N., Woldeamanuel, Y., Asrat, D., and Debella, A. (2014). Effect of Cymbopogon martinii, Foeniculum vulgare, and Trachyspermum ammi essential oils on the growth and mycotoxins production by Aspergillus species. Int. J. Food Sci. 874135, 1–9.
Gil-Serna, J., Mateo, E. M., Gonzalez-Jaen, M. T., Jimenez, M., Vazquez, C., and Patino, B. (2013). Contamination of barley seeds with Fusarium species and their toxins in Spain: an integrated approach. Food Addit. Contam. Part A 30, 372–380. doi: 10.1080/19440049.2012.743040
Giray, B., Atasayar, S., and Sahin, G. (2009). Determination of ochratoxin A and total aflatoxin levels in corn samples from Turkey by enzyme-linked immunosorbent assay. Mycotoxin Res. 25, 113–116. doi: 10.1007/s12550-009-0016-0
Giray, B., Girgin, G., Engin, A. B., Aydın, S., and Sahin, G. (2007). Aflatoxin levels in wheat samples consumed in some regions of Turkey. Food Control 18, 23–29. doi: 10.1016/j.foodcont.2005.08.002
Gomori, C., Nacsa-Farkas, E., Kerekes, E. B., Kocsube, S., Vagvolgyi, C., and Krisch, J. (2013). Evaluation of five essential oils for the control of food spoilage and mycotoxin producing fungi. Acta Biol. Szegediensis 57, 113–116.
Gonçalez, E., Nogueira, J. H. C., Fonseca, H., Felicio, J. D., Pino, F. A., and Correa, B. (2008). Mycobiota and mycotoxins in Brazilian peanut kernels from sowing to harvest. Int. J. Food Microbiol. 123, 184–190. doi: 10.1016/j.ijfoodmicro.2008.01.012
Gonzalez-Jartin, J. M., Alfonso, A., Rodriguez, I., Sainz, M. J., Vieytes, M. R., and Botana, L. M. (2019). A QuEChERS based extraction procedure coupled to UPLC-MS/ MS detection for mycotoxins analysis in beer. Food Chem. 275, 703–710. doi: 10.1016/j. foodchem.2018.09.162
Goud, K. Y., Reddy, K. K., Satyanarayana, M., Kummari, S., and Gobi, K. V. (2020). A review on recent developments in optical and electrochemical aptamer-based assays for mycotoxins using advanced nanomaterials. Microchim. Acta 187:29. doi: 10.1007/ s00604-019-4034-0
Gummert, M., Balingbing, C. B., Barry, G., and Estevez, L. A. (2009). Management options, technologies and strategies for minimized mycotoxin contamination of rice. World Mycotoxin J. 2, 151–159. doi: 10.3920/WMJ2008.1131
Guo, P., Yang, W., Hu, H., Wang, Y., and Li, P. (2019). Rapid detection of aflatoxin B1 by dummy template molecularly imprinted polymer capped CdTe quantum dots. Anal. Bioanal. Chem. 411, 2607–2617. doi: 10.1007/s00216-019-01708-2
Gurney, S. M. R., Scott, K. S., Kacinko, S. L., Presley, B. C., and Logan, B. K. (2014). Pharmacology, toxicology, and adverse effects of synthetic cannabinoid drugs. Forensic Sci. Rev. 26:53.
Han, Z., Jiang, K., Fan, Z., Diana Di Mavungu, J., Dong, M., Guo, W., et al. (2017). Multi-walled carbon nanotubes-based magnetic solid-phase extraction for the determination of zearalenone and its derivatives in maize by ultra-high performance liquid chromatography-tandem mass spectrometry. Food Control 79, 177–184. doi: 10.1016/j.foodcont.2017.03.044
Harris, K. L., Bobe, G., and Bourquin, L. D. (2009). Patulin surveillance in apple cider and juice marketed in Michigan. J. Food Prot. 72, 1255–1261. doi: 10.4315/0362- 028X-72.6.1255
Hassan, Z. U., Al Thani, R., Balmas, V., Migheli, Q., and Jaoua, S. (2019). Prevalence of Fusarium fungi and their toxins in marketed feed. Food Control 104, 224–230. doi: 10.1016/j.foodcont.2019.04.045
Hell, K., Cardwell, K. F., and Poehling, H. M. (2003). Distribution of fungal species and aflatoxin contamination in stored maize in four agroecological zones in Benin, West-Africa. J. Phytopathol. 151, 690–698. doi: 10.1046/j.1439-0434.2003.00792.x
Hell, K., Setamou, M., Cardwell, K. F., and Poehling, H. M. (2000). The influence of storage practices on aflatoxin contamination in maize in four agroecological zones in Benin, West Africa. J. Stored Prod. Res. 36, 365–382. doi: 10.1016/S0022-474X(99)00056-9
Hendrickson, O. D., Chertovich, J. O., Zherdev, A. V., Sveshnikov, P. G., and Dzantiev, B. B. (2018). Ultrasensitive magnetic ELISA of zearalenone with preconcentration and chemiluminescent detection. Food Control 84, 330–338. doi: 10.1016/j.foodcont.2017.08.008
Hope, R., Cairns-Fuller, V., Aldred, D., and Magan, N. (2005). Use of antioxidants and essential oils for controlling mycotoxins in grain. BCPC Crop Sci. Technol. 5, 429–436.
Houissa, H., Lasram, S., Sulyok, M., Šarkanj, B., Fontana, A., Strub, C., et al. (2019). Multimycotoxin LC-MS/MS analysis in pearl millet (Pennisetum glaucum) from Tunisia. Food Control 106:106738. doi: 10.1016/j.foodcont.2019.106738
Huang, Z., He, J., Li, H., Zhang, M., Wang, H., Zhang, Y., et al. (2020). Synthesis and application of magnetic-surfaced pseudo molecularly imprinted polymers for zearalenone pretreatment in cereal samples. Food Chem. 308:125696. doi: 10.1016/j. foodchem.2019.125696
Huang, X., Tang, X., Jallow, A., Qi, X., Zhang, W., Jiang, J., et al. (2020). Development of an ultrasensitive and rapid fluorescence polarization immunoassay for Ochratoxin A in Rice. Toxins 12:682. doi: 10.3390/toxins12110682
Huertas-Perez, J. F., Arroyo-Manzanares, N., Garcia-Campana, A. M., and Gamiz-Gracia, L. (2017). Solid phase extraction as sample treatment for the determination of Ochratoxin A in foods: a review. Crit. Rev. Food Sci. Nutr. 57, 3405–3420. doi: 10.1080/10408398.2015.1126548
Hussain, S., Asi, M. R., Iqbal, M., Khalid, N., Wajih-ul-Hassan, S., and Ariño, A. (2020). Patulin mycotoxin in mango and orange fruits, juices, pulps, and jams marketed in Pakistan. Toxins 12:52. doi: 10.3390/toxins12010052
Hussaini, A. M., Timothy, A. G., Olufunmilayo, H. A., Akanya, H., Ezekiel, A. S., and Ogbadu, G. H. (2009a). Fungi and some mycotoxins contaminating rice (Oryza sativa) in Niger State, Nigeria. Afr. J. Biotechnol. 6, 099–108.
Hussaini, A. M., Timothy, A. G., Olufunmilayo, H. A., Ezekiel, A. S., and Godwin, H. O. (2009b). Fungi and some mycotoxins found in mouldy sorghum in Niger state, Nigeria. World J. Agric. Sci. 5, 05–17.
Icli, N. (2019). Occurrence of patulin and 5-hydroxymethylfurfural in apple sour, which is a traditional product of Kastamonu, Turkey. Food Addit. Contam. Part A 36, 952–963. doi: 10.1080/19440049.2019.1605207
Iqbal, S. Z., Asi, M. R., Hanif, U., Zuber, M., and Jinap, S. (2016). The presence of aflatoxins and ochratoxin A in rice and rice products; and evaluation of dietary intake. Food Chem. 210, 135–140. doi: 10.1016/j.foodchem.2016.04.104
Iqbal, S. Z., Malik, S., Asi, M. R., Selamat, J., and Malik, N. (2018). Natural occurrence of patulin in different fruits, juices and smoothies and evaluation of dietary intake in Punjab, Pakistan. Food Control 84, 370–374. doi: 10.1016/j.foodcont.2017.08.024
Irakli, M. N., Skendi, A., and Papageorgiou, M. D. (2017). HPLC-DAD-FLD method for simultaneous determination of mycotoxins in wheat bran. J. Chromatogr. Sci. 55, 690–696. doi: 10.1093/chromsci/bmx022
Iram, W., Anjum, T., Iqbal, M., Ghaffar, A., Abbas, M., and Khan, A. M. (2016). Structural analysis and biological toxicity of aflatoxins B1 and B2 degradation products following detoxification by Ocimum basilicum and Cassia fistula aqueous extracts. Front. Microbiol. 7, 1–18. doi: 10.3389/fmicb.2016.01105
James, B., Adda, C., Cardwell, K., Annang, D., Hell, K., Korie, S., et al. (2007). Public information campaign on aflatoxin contamination of maize grains in market stores in Benin, Ghana and Togo. Food Addit. Contam. 24, 1283–1291. doi: 10.1080/02652030701416558
Janardhana, G. R., Raveesha, K. A., and Shetty, H. S. (1999). Mycotoxin contamination of maize grains grown in Karnataka (India). Food Chem. Toxicol. 37, 863–868. doi: 10.1016/S0278-6915(99)00067-8
Janik, E., Niemcewicz, M., Podogrocki, M., Ceremuga, M., Gorniak, L., Stela, M., et al. (2021). The existing methods and novel approaches in mycotoxins detection. Molecules 26:3981. doi: 10.3390/molecules26133981
Jayaraman, P., and Kalyanasundaram, I. (1990). Natural occurrence of toxigenic fungi and mycotoxins in rice bran. Mycopathologia 110, 81–85. doi: 10.1007/BF00446995
Jestoi, M. (2008). Emerging fusarium-mycotoxins fusaproliferin, beauvericin, enniatins, and Moniliformin-A review. Crit. Rev. Food Sci. Nutr. 48, 21–49. doi: 10.1080/10408390601062021
Ji, F., He, D., Olaniran, A., Mokoena, M., Xu, J., and Shi, J. (2019). Occurrence, toxicity, production and detection of Fusarium mycotoxin: A review. Food Prod. Proc. Nutr. 1:6. doi: 10.1186/s43014-019-0007-2
Jiang, D., Wei, D., Wang, L., Ma, S., Du, Y., and Wang, M. (2018). Multi walled carbon nanotube for one-step cleanup of 21 mycotoxins in corn and wheat prior to ultraperformance liquid chromatography-tandem mass spectrometry analysis. Toxins 10:409. doi: 10.3390/toxins10100409
Jimenez, M., and Mateo, R. (1997). Determination of mycotoxins produced by Fusarium isolates from banana fruits by capillary gas chromatography and highperformance liquid chromatography. J. Chromatogr. A 778, 363–372. doi: 10.1016/ S0021-9673(97)00328-2
Juan, C., Mañes, J., Font, G., and Juan-Garcia, A. (2017). Determination of mycotoxins in fruit berry by-products using QuEChERS extraction method. LWT 86, 344–351. doi: 10.1016/j.lwt.2017.08.020
Jurjevic, J., Solfrizzo, M., Cvjetkovic, B., De Girolamo, A., and Visconti, A. (2002). Occurrence of Beauvericin in corn from Croatia. Food Technol. Biotechnol. 40, 91–94.
Jurjevic, Z., Wilson, J. P., Wilson, D. M., and Casper, H. H. (2007). Changes in fungi and mycotoxins in pearl millet under controlled storage conditions. Mycopathologia 164, 229–239. doi: 10.1007/s11046-007-9042-7
Kaaya, A. N., Eigel, W., and Harris, C. (2006). Peanut aflatoxin levels on farms and in markets of Uganda. Peanut Sci. 33, 68–75. doi: 10.3146/0095-3679(2006)33[68:PALOFA] 2.0.CO;2
Karoki, P. K., Njue, W. M., Swaleh, S., Njoroge, E. K., and Kathurima, C. W. (2018). Determination of Ochratoxin A in selected cereal grains retailed in Nairobi County, Kenya. J. Food Res. 7:79. doi: 10.5539/jfr.v7n5p79
Khodaei, D., Javanmardi, F., and Khaneghah, A. M. (2021). The global overview of the occurrence of mycotoxins in cereals: a three-year survey. Curr. Opin. Food Sci. 39, 36–42. doi: 10.1016/j.cofs.2020.12.012
Kishore, G. K., Pande, S., Manjula, K., Rao, J. N., and Thomas, D. (2002). Occurrence of mycotoxins and toxigenic fungi in groundnut (Arachis hypogaea L.) seeds in Andhra Pradesh, India. Plant Pathol. J. 18, 204–209. doi: 10.5423/PPJ.2002.18.4.204
Kos, J., JanićHajnal, E., Malachová, A., Steiner, D., Stranska, M., Krska, R., et al. (2020). Mycotoxins in maize harvested in republic of Serbia in the period 2012–2015. Part 1: regulated mycotoxins and its derivatives. Food Chem. 312:126034. doi: 10.1016/j. foodchem.2019.126034
Kritzinger, Q., Aveling, T. A., Marasas, W. F., Rheeder, J. P., Van Der Westhuizen, L., and Shephard, G. S. (2002). Mycoflora and fumonisin mycotoxins associated with cowpea (Vigna unguiculata (L.) Walp) seeds. J. Agric. Food Chem. 51, 2188–2192. doi: 10.1021/jf026121v
Krska, R., Welzig, E., Berthiller, F., Molinelli, A., and Mizaikoff, B. (2005). Advances in the analysis of mycotoxins and its quality assurance. Food Addit. Contam. 22, 345–353. doi: 10.1080/02652030500070192
Kuiper-Goodman, T., Scott, P. M., and Watanabe, H. (1987). Risk assessment of the mycotoxin Zearalenone. Regul. Toxicol. Pharmacol. 7, 253–306. doi: 10.1016/0273-2300(87)90037-7
Kunz, B. M., Wanko, F., Kemmlein, S., Bahlmann, A., Rohn, S., and Maul, R. (2020). Development of a rapid multi-mycotoxin LC-MS/MS stable isotope dilution analysis for grain legumes and its application on 66 market samples. Food Control 109:106949. doi: 10.1016/j.foodcont.2019.106949
Lahouar, A., Jedidi, I., Sanchis, V., and Saïd, S. (2018). Aflatoxin B1, ochratoxin A and zearalenone in sorghum grains marketed in Tunisia. Food Addi. Contam. Part B Surv. 11, 103–110. doi: 10.1080/19393210.2018.1433239
Laidou, I. A., Thanassoulopoulos, C. C., and Liakopoulou-Kyriakides, M. (2001). Diffusion of patulin in the flesh of pears inoculated with four post-harvest pathogens. J. Phytopathol. 149, 457–461. doi: 10.1046/j.1439-0434.2001.00663.x
Lattanzio, V. M. T., Ciasca, B., Powers, S., and Visconti, A. (2014). Improved method for the simultaneous determination of aflatoxins, ochratoxin A and Fusarium toxins in cereals and derived products by liquid chromatography–tandem mass spectrometry after multi-toxinimmuno affinity clean up. J. Chromatogr. A 1354, 139–143. doi: 10.1016/j.chroma.2014.05.069
Lattanzio, V. M., Solfrizzo, M., Powers, S., and Visconti, A. (2007). Simultaneous determination of aflatoxins, ochratoxin A and Fusarium toxins in maize by liquid chromatography/tandem mass spectrometry after multitoxin immunoaffinity cleanup. Rapid Commun. Mass Spectrom. 21, 3253–3261. doi: 10.1002/rcm.3210
Lattanzio, V. M. T., Von Holst, C., Lippolis, V., De Girolamo, A., Logrieco, A. F., Mol, H. G. J., et al. (2019). Evaluation of mycotoxin screening tests in a verification study involving first time users. Toxins 11:129. doi: 10.3390/toxins11020129
Le, V. T., Vasseghian, Y., Dragoi, E. N., Moradi, M., and Mousavi Khaneghah, A. (2021). A review on graphene-based electrochemical sensor for mycotoxins detection. Food Chem. Toxicol. 148:111931. doi: 10.1016/j.fct.2020.111931
Lee, H. J., and Ryu, D. (2017). Worldwide occurrence of mycotoxins in cereals and cereal-derived food products: public health perspectives of their co-occurrence. J. Agric. Food Chem. 65, 7034–7051. doi: 10.1021/acs.jafc.6b04847
Lee, T. P., Sakai, R., Abdul Manaf, N., MohdRodhi, A., and Saad, B. (2014). High performance liquid chromatography method for the determination of patulin and 5-hydroxymethylfurfural in fruit juices marketed in Malaysia. Food Control 38, 142–149. doi: 10.1016/j.foodcont.2013.10.018
Li, Y., and Beghin, J. C. (2014). Protectionism indices for non-tariff measures: an application to maximum residue levels. Food Policy 45, 57–68. doi: 10.1016/j. foodpol.2013.12.005
Lippolis, V., Cervellieri, S., Damascelli, A., Pascale, M., Di Gioia, A., Longobardi, F., et al. (2018). Rapid prediction of deoxynivalenol contamination in wheat bran by MOSbased electronic nose and characterization of the relevant pattern of volatile compounds. J. Sci. Food Agric. 98, 4955–4962. doi: 10.1002/jsfa.9028
Lippolis, V., Pascale, M., Cervellieri, S., Damascelli, A., and Visconti, A. (2014). Screening of deoxynivalenol contamination in durum wheat by MOS-based electronic nose and identification of the relevant pattern of volatile compounds. Food Control 37, 263–271. doi: 10.1016/j.foodcont.2013.09.048
Liu, Z., Gao, J., and Yu, J. (2006). Aflatoxins in stored maize and rice grains in Liaoning province, China. J. Stored Prod. Res. 42, 468–479. doi: 10.1016/j.jspr.2005.09.003
Liu, X., Liu, X., Huang, P., Wei, F., Ying, G., Lu, J., et al. (2018). Regeneration and reuse of immunoaffinity column for highly efficient clean-up and economic detection of ochratoxin a in malt and ginger. Toxins 10:462. doi: 10.3390/toxins10110462
Loo, J. F. C., Chien, Y. H., Yin, F., Kong, S. K., Ho, H. P., and Yong, K. T. (2019). Upconversion and downconversion nanoparticles for biophotonics and nanomedicine. Coord. Chem. Rev. 400:213042. doi: 10.1016/j.ccr.2019.213042
Lopez, A. G., Theumer, M. G., Zygadlo, J. A., and Rubinstein, H. R. (2004). Aromatic plants essential oils activity on Fusarium verticillioides Fumonisin B1 production in corn grain. Mycopathologia 158, 343–349. doi: 10.1007/s11046-005-3969-3
MacDonald, S., Wilson, P., Barnes, K., Damant, A., Massey, R., Mortby, E., et al. (1999). Ochratoxin A in dried vine fruit: method development and survey. Food Addit. Contam. 16, 253–260. doi: 10.1080/026520399284019
Magan, N. (2006). Mycotoxins in Europe: prevention and early detection strategies. Mycopathologia 162, 245–253. doi: 10.1007/s11046-006-0057-2
Magnoli, C., Astoreca, A., Ponsone, L., Combina, M., Palacio, G., Rosa, C. A. R., et al. (2004). Survey of mycoflora and ochratoxin A in dried vine fruits from Argentina markets. Lett. Appl. Microbiol. 39, 326–331. doi: 10.1111/j.1472-765X.2004.01583.x
Magnoli, C., Astoreca, A., Ponsone, M. L., Fernández-Juri, M. G., Barberis, G., and Dalcero, A. M. (2007). Ochratoxin A and Aspergillus section Nigri in peanut seeds at different months of storage in Córdoba, Argentina. Int. J. Food Microbiol. 119, 213–218. doi: 10.1016/j.ijfoodmicro.2007.07.056
Magnoli, C., Astoreca, A., Ponsone, L., Fernández-Juri, M. G., Chiacchiera, S., and Dalcero, A. (2006). Ochratoxin A and the occurrence of ochratoxin A-producing black aspergilli in stored peanut seeds from Córdoba, Argentina. J. Sci. Food Agric. 86, 2369–2373. doi: 10.1002/jsfa.2625
Majdinasaba, M., Sheikh-Zeinoddin, M., Soleimanian-Zad, S., Li, P., Zhang, Q., Li, X., et al. (2015). Ultrasensitive and quantitative gold nanoparticle-based immunochromatographic assay for detection of ochratoxin A in agro-products. J. Chromatogr. B 974, 147–154. doi: 10.1016/j.jchromb.2014.10.034
Majeed, S., Boevre, M. D., Saeger, S. D., Rauf, W., Tawab, A., Habib, F., et al. (2018). Multiple mycotoxins in rice: occurrence and health risk assessment in children and adults of Punjab, Pakistan. Toxins 10:77. doi: 10.3390/toxins10020077
Majeed, S., Iqbal, M., Asi, M. R., and Iqbal, S. Z. (2013). Aflatoxins and ochratoxin A contamination in rice, corn and corn products from Punjab, Pakistan. J. Cereal Sci. 58, 446–450. doi: 10.1016/j.jcs.2013.09.007
Majid, I. (2007). Studying the mycotoxins found in wheat and maize under conditions of storage. Ph.D. Dissertation. Damascus University, Syria.
Makun, H. A., Dutton, M. F., Njobeh, P. B., Mwanza, M., and Kabiru, A. Y. (2011). Natural multi occurrence of mycotoxins in rice from Niger state, Nigeria. Mycotoxin Res. 27, 97–104. doi: 10.1007/s12550-010-0080-5
Malachova, A., Stranska, M., Vaclavikova, M., Elliott, C. T., Black, C., Meneely, J., et al. (2018). Advanced LC–MS-based methods to study the co-occurrence and metabolization of multiple mycotoxins in cereals and cereal-based food. Anal. Bioanal. Chem. 410, 801–825. doi: 10.1007/s00216-017-0750-7
Mallmann, C.A., Dilkin, M., Mürmann, L., Dilkin, P., and Almeida, C.A.A. (2003). Avaliação da contaminação por deoxivalenol em trigo utilizado na alimentação humana. In: Congresso Brasileiro de Farmácia. Anais.
Maraqa, A., Alsharoa, N. F., Farah, H., Albjeirami, W. M., Shakya, A. K., and Sallal, A. J. (2007). Effect of Nigella sativa extract and oil on aflatoxin production by Aspergillus flavus. Turk. J. Biol. 31, 155–159.
Marin, S., Ramos, A. J., Ho, G. C., and Sanchis, V. (2012). Reduction of mycotoxins and toxigenic fungi in the Mediterranean basin maize chain. Phytopathol. Mediterr. 51, 93–118.
Marin, S., Velluti, A., Ramos, A. J., and Sanchis, V. (2004). Effect of essential oils on zearalenone and deoxynivalenol production by Fusarium graminearum in non-sterilized maize grain. Food Microbiol. 21, 313–318. doi: 10.1016/j.fm.2003.08.002
Martins, M. L., Gimeno, A., Martins, H. M., and Bernardo, F. (2002). Co-occurrence of patulin and citrinin in Portuguese apples with rotten spots. Food Addit. Contam. 19, 568–574. doi: 10.1080/02652030210121320
Mateus, A. R. S., Barros, S., Pena, A., and Sanches Silva, A. (2021). Mycotoxins in pistachios (Pistacia vera L.): methods for determination, occurrence, decontamination. Toxins 13:682. doi: 10.3390/toxins13100682
Matumba, L., Van Poucke, C., Monjerezi, M., NjumbeEdiage, E., and De Saeger, S. (2015). Concentrating aflatoxins on the domestic market through groundnut export: A focus on Malawian groundnut value and supply chain. Food Control 51, 236–239. doi: 10.1016/j.foodcont.2014.11.035
Mazumder, P. M., and Sasmal, D. (2001). Mycotoxins–limits and regulations. Anc. Sci. Life 20:1.
Mehrez, A., Maatouk, I., Romero-González, R., Amara, A. B., Kraiem, M., Frenich, A. G., et al. (2016). Assessment of ochratoxin A stability following gamma irradiation: experimental approaches for feed detoxification perspectives. World Mycotoxin J. 9, 289–298. doi: 10.3920/WMJ2013.1652
Melinte, G., Hosu, O., Cristea, C., and Marrazza, G. (2022). DNA sensing technology a useful food scanning tool. Trends Analytic. Chem. 154:116679. doi: 10.1016/j. trac.2022.116679
Mendez-Albores, A., Arambula-Villa, G., Loarca-Pina, M. G. F., Castano-Tostado, E., and Moreno-Martínez, E. (2005). Safety and efficacy evaluation of aqueous citric acid to degrade B-aflatoxins in maize. Food Chem. Toxicol. 43, 233–238. doi: 10.1016/j. fct.2004.09.009
Miklos, G., Angeli, C., Ambrus, A., Nagy, A., Kardos, V., Zentai, A., et al. (2020). Detection of aflatoxins in different matrices and food-chain positions. Front. Microbiol. 11:1916. doi: 10.3389/fmicb.2020.01916
Mir, S. A., Dar, B. N., Shah, M. A., Sofi, S. A., Hamdani, A. M., Oliveira, C. A. F., et al. (2021). Application of new technologies in decontamination of mycotoxins in cereal grains: challenges, and perspectives. Food Chem. Toxicol. 148:111976. doi: 10.1016/j.fct.2021.111976
Mogensen, J. M., Thrane, J. C., and Nielsen, K. F. (2010). Production of fumonisin B2 and B4 by Aspergillus niger on grapes and raisins. J. Agric. Food Chem. 58, 954–958. doi: 10.1021/jf903116q
Mohamed, S. R., Deabes, M. M., Abdel-Motaleb, N. M., Mohamed, S. S., Badr, A. N., Shaban, E. M., et al. (2012). Antifungal activity of basil and mustard essential oils against spoilage toxigenic fungi in Egyptian pan bread and its economic evaluation. J. Appl. Sci. Res. 8, 5536–5542.
Mohamed, N. F., El-Dine, R. S. S., Kotb, M. A. M., and Saber, A. (2015). Assessing the possible effect of gamma irradiation on the reduction of aflatoxin B1, and on the moisture content in some cereal grains. Am. J. Biomed. Sci. 7, 33–39.
Mohana, D. C., Thippeswamy, S., Abhishek, R. U., Shobha, B., and Mamatha, M. G. (2017). Studies on seed-borne mycoflora and aflatoxin B1 contaminations in food-based seed samples: molecular detection of mycotoxigenic Aspergillus flavus and their management. Int. Food Res. J. 24, 422–427.
Moukas, A., Panagiotopoulou, V., and Markaki, P. (2008). Determination of patulin in fruit juices using HPLCDAD and GCMSD techniques. Food Chem. 109, 860–867. doi: 10.1016/j.foodchem.2008.01.015
Mravlje, J., Regvar, M., Staric, P., Mozetic, M., and Vogel-Mikus, K. (2021). Cold plasma affects germination and fungal community structure of buckwheat seeds. Plan. Theory 10:851. doi: 10.3390/plants10050851
Muriuki, G. K., and Siboe, G. M. (1995). Maize flour contaminated with toxigenic fungi and mycotoxins in Kenya. Afr. J. Health Sci. 2, 236–241.
Murthy, P. S., Ramalakshmi, K., and Srinivas, P. (2009). Fungitoxic activity of Indian borage (Plectranthus amboinicus) volatiles. Food Chem. 114, 1014–1018. doi: 10.1016/j. foodchem.2008.10.064
Muthomi, , and Mutitu, (2003). Occurrence of mycotoxin producing Fusarium species and other fungi on wheat kernels harvested in selected districts of Kenya. Afri. Crop Sci. Conf. Proc. 6, 335–339.
Nakagawa, H., Ohmichi, K., Sakamoto, S., Sago, Y., Kushiro, M., Nagashima, H., et al. (2011). Detection of a new Fusarium masked mycotoxin in wheat grain by highresolution LC–Orbitrap™ MS. Food Addit. Contam: Part A 28, 1447–1456. doi: 10.1080/19440049.2011.597434
Nascimento, M. S., and Taniwaki, M. H. (2023). “Common and natural occurrence of pathogens, including fungi, leading to primary and secondary product contamination” in Present knowledge in food safety. eds. M. E. Knowles, L. E. Anelich, A. R. Boobis and B. Popping (Greece: Elsevier), 330–356.
Nazir, A., Kalim, I., Sajjad, M., Usman, M., and Iqbal, M. (2019). Prevalence of aflatoxin contamination in pulses and spices in different regions of Punjab. Chem. Int. 5, 274–280.
Nganou Donkeng, N., SokamteTegang, A., TchindaSonwa, E., NtsamoBeumo, T. M., Nodem Sohanang, F. S., Douanla Nodem, N. F., et al. (2022). Fungal diversity and occurrence of Aflatoxin B1, Citrinine, and Ochratoxin A in rice of Cameroon. J. Food Proc. Preserve. 46:e16429. doi: 10.1111/jfpp.16429
Niyibituronsa, M., Onyango, A. N., Gaidashova, S., Imathiu, S. M., Uwizerwa, M., Wanjuki, I., et al. (2018). Evaluation of mycotoxin content in soybean (Glycine max L.) grown in Rwanda. Afr. J. Food Agric. Nutr. Dev. 18, 13808–13824. doi: 10.18697/ ajfand.83.17710
Olatunde, O. O., Shiekh, K. A., and Benjakul, S. (2021). Pros and cons of cold plasma technology as an alternative non-thermal processing technology in seafood industry. Trends Food Sci. Technol. 111, 617–627. doi: 10.1016/j.tifs.2021.03.026
Onyedum, S. C., Adefolalu, F. S., Muhammad, H. L., Apeh, D. O., Agada, M. S., Imienwanrin, M. R., et al. (2020). Occurrence of major mycotoxins and their dietary exposure in north-Central Nigeria staples. Sci. African 7:e00188. doi: 10.1016/j. sciaf.2019.e00188
Orlando, B., Grignon, G., Vitry, C., Kashefifard, K., and Valade, R. (2019). Fusarium species and enniatin mycotoxins in wheat, durum wheat, triticale and barley harvested in France. Mycotoxin Res. 35, 369–380. doi: 10.1007/s12550-019-00363-x
Oruc, H. H., Cengiz, M., and Kalkanli, O. (2006). Comparison of aflatoxin and fumonisin levels in maize grown in Turkey and imported from the USA. Anim. Feed Sci. Technol. 128, 337–341. doi: 10.1016/j.anifeedsci.2006.02.008
Oteiza, J. M., Khaneghah, A. M., Campagnollo, F. B., Granato, D., Mahmoudi, M. R., Sant’Ana, A. S., et al. (2017). Influence of production on the presence of patulin and ochratoxin A in fruit juices and wines of Argentina. LWT Food Sci. Technol. 80, 200–207. doi: 10.1016/j.lwt.2017.02.025
Ott, L. C., Appleton, H. J., Shi, H., Keener, K., and Mellata, M. (2021). High voltage atmospheric cold plasma treatment inactivates Aspergillus flavus spores and deoxynivalenol toxin. Food Microbiol. 95:103669. doi: 10.1016/j.fm.2020.103669
Ottoboni, M., Pinotti, L., Tretola, M., Giromini, C., Fusi, E., Rebucci, R., et al. (2018). Combining E-nose and lateral flow immunoassays (LFIAs) for rapid occurrence/cooccurrence aflatoxin and fumonisin detection in maize. Toxins 10:416. doi: 10.3390/ toxins10100416
Oyedele, O. A., Ezekiel, C. N., Sulyok, M., Adetunji, M. C., Warth, B., Atanda, O. O., et al. (2017). Mycotoxin risk assessment for consumers of groundnut in domestic markets in Nigeria. Int. J. Food Microbiol. 251, 24–32. doi: 10.1016/j. ijfoodmicro.2017.03.020
Palumbo, J. D., O’Keeffe, T. L., Vasquez, S. J., and Mahoney, N. E. (2011). Isolation and identification of ochratoxin A-producing Aspergillus section Nigri strains from California raisins. Lett. Appl. Microbiol. 52, 330–336. doi: 10.1111/j.1472-765X.2011.03004.x
Pande, N., Saxena, J., and Pandey, H. (1990). Natural occurrence of mycotoxins in some cereals. Mycoses 33, 126–128. doi: 10.1111/myc.1990.33.3.126
Pandey, A. K., Samota, M. K., and Silva, A. S. (2022). Mycotoxins along the tea supply chain: a dark side of an ancient and high valued aromatic beverage. Crit. Rev. Food Sci. Nutr. doi: 10.1080/10408398.2022.2061908
Pandey, A. K., Sonker, N., and Singh, P. (2016). Efficacy of some essential oils against Aspergillus flavus with special reference to Lippia alba oil an inhibitor of fungal proliferation and aflatoxin B1 production in green gram seeds during storage. J. Food Sci. Technol. 81, M928–M934. doi: 10.1111/1750-3841.13254
Pascale, M., De Girolamo, A., Lippolis, V., Stroka, J., Mol, H. G. J., and Lattanzio, V. M. T. (2019). Performance evaluation of LC-MS methods for multimycotoxin determination. J. AOAC Int. 102, 1708–1720. doi: 10.5740/jaoacint.19-0068
Payne, G. A., and Brown, M. P. (1998). Genetics and physiology of aflatoxin biosynthesis. Annu. Rev. Phytopathol. 36, 329–362. doi: 10.1146/annurev.phyto.36.1.329
Pereira, V. L., Fernandes, J. O., and Cunha, S. C. (2014). Mycotoxins in cereals and related foodstuffs: A review on occurrence and recent methods of analysis. Trends Food Sci. Technol. 36, 96–136. doi: 10.1016/j.tifs.2014.01.005
Pernica, M., Kyralová, B., Svoboda, Z., Boško, R., Brožková, I., Česlová, L., et al. (2022). Levels of T-2 toxin and its metabolites, and the occurrence of Fusarium fungi in spring barley in the Czech Republic. Food Microbiol. 102:103875. doi: 10.1016/j. fm.2021.103875
Pietri, A., Bertuzzi, T., Pallaroni, L., and Piva, G. (2004). Occurance of mycotoxins and ergosterol in maize harvested over 5 years in the northern Italy. Food Addit. Contam. 21, 479–487. doi: 10.1080/02652030410001662020
Pildain, M. B., Vaamonde, G., and Cabral, D. (2004). Analysis of population structure of Aspergillus flavus from peanut based on vegetative compatibility, geographic origin, mycotoxin and sclerotia production. Int. J. Food Microbiol. 93, 31–40. doi: 10.1016/j. ijfoodmicro.2003.10.007
Polišenská, I., Jirsa, O., Vaculová, K., Pospíchalová, M., Wawroszova, S., and Frydrych, J. (2020). Fusarium mycotoxins in two hulless oat and barley cultivars used for food purposes. Foods 9:1037. doi: 10.3390/foods9081037
Ponzilacqua, B., Rottinghaus, G. E., Landers, B. R., and Oliveira, C. A. F. (2019). Effects of medicinal herb and Brazilian traditional plant extracts on in vitro mycotoxin decontamination. Food Control 100, 24–27. doi: 10.1016/j.foodcont.2019.01.009
Prakash, B., Shukla, S., Singh, P., Mishra, P. K., Dubey, N. K., and Kharwar, R. N. (2011). Efficacy of chemically characterized Ocimum gratissimum L. essential oil as an antioxidant and a safe plant-based antimicrobial against fungal and aflatoxin B1 contamination of spices. Food Res. Int. 44, 385–390. doi: 10.1016/j.foodres.2010.10.002
Puel, O., Galtier, P., and Oswald, I. P. (2010). Biosynthesis and toxicological effects of patulin. Toxins 2, 613–631. doi: 10.3390/toxins2040613
Rai, A., Dixit, S., Singh, S. P., Gautam, N. K., Das, M., and Tripathi, A. (2018). Presence of zearalenone in cereal grains and its exposure risk assessment in Indian population. J. Food Sci. 83, 3126–3133. doi: 10.1111/1750-3841.14404
Rasooli, I., Fakoor, M. H., Yadegarinia, D., Gachkar, L., Allameh, A., and Rezaei, M. B. (2008). Anti mycotoxigenic characteristics of Rosmarinus officinalis and Trachyspermum copticum L. essential oils. Int. J. Food Microbiol. 122, 135–139. doi: 10.1016/j. ijfoodmicro.2007.11.048
Rasooli, I., and Owlia, P. (2005). Chemoprevention by thyme oils of Aspergillus parasiticus growth and aflatoxin production. Phytochemistry 66, 2851–2856. doi: 10.1016/j.phytochem.2005.09.029
Razzaghi-Abyaneh, M., Yoshinari, T., Shams-Ghahfarokhi, M., Rezaee, M. B., Nagasawa, H., and Sakuda, S. (2007). Dillapiol and apiolas specific inhibitors for the biosynthesis of aflatoxin G1 in Aspergillus parasiticus. Biosci. Biotechnol. Biochem. 71, 2329–2332. doi: 10.1271/bbb.70264
Reddy, K. R. N., Reddy, C. S., and Muralidharan, K. (2009a). Potential of botanicals and biocontrol agents on growth and aflatoxin production by Aspergillus flavus infecting rice grains. Food Control. 20, 173–178. doi: 10.1016/j.foodcont.2008.03.009
Reddy, K. R., Reddy, C. S., and Muralidharan, K. (2009b). Detection of Aspergillus spp. and aflatoxin B1 in rice in India. Food Microbiol. 26, 27–31. doi: 10.1016/j.fm.2008.07.013
Richard, J. L., Plattner, R. D., Mary, J., and Liska, S. L. (1999). The occurrence of Ochratoxin A in dust collected from a problem home hold. Mycopathologia 146, 99–103. doi: 10.1023/A:1007056627296
Rico-Yuste, A., Gomez-Arribas, L. N., Perez-Conde, M. C., Urraca, J. L., and Moreno-Bondi, M. C. (2018). Rapid determination of Alternaria mycotoxins in tomato samples by pressurized liquid extraction coupled to liquid chromatography with fluorescence detection. Food Addit. Contam. Part A 35, 2175–2182. doi: 10.1080/19440049.2018.1512759
Rubert, J., Dzuman, Z., Vaclavikova, M., Zachariasova, M., Soler, C., and Hajslova, J. (2012). Analysis of mycotoxins in barley using ultra high liquid chromatography high resolution mass spectrometry: comparison of efficiency and efficacy of different extraction procedures. Talanta 99, 712–719. doi: 10.1016/j.talanta.2012.07.010
Savi, G. D., Piacentini, K. C., Bittencourt, K. O., and Scussel, V. M. (2014). Ozone treatment efficiency on Fusarium graminearum and deoxynivalenol degradation and its effects on whole wheat grains (Triticum aestivum L.) quality and germination. J. Stored Prod. Res. 59, 245–253. doi: 10.1016/j.jspr.2014.03.008
Savi, G. D., Piacentini, K. C., and Scussel, V. M. (2015). Ozone treatment efficiency in Aspergillus and Penicillium growth inhibition and mycotoxin degradation of stored wheat grains (Triticum aestivum L.). J. Food Process. Preserv. 39, 940–948. doi: 10.1111/ jfpp.12307
Saxena, N., Dwivedi, P. D., Ansari, K. M., and Das, M. (2008). Patulin in apple juices: incidence and likely intake in an Indian population. Food Addit. Contam. 1, 140–146. doi: 10.1080/02652030802378848
Schöneberg, T., Martin, C., Wettstein, F. E., Bucheli, T. D., Mascher, F., Bertossa, M., et al. (2016). Fusarium and mycotoxin spectra in Swiss barley are affected by various cropping techniques. Food Addit. Contam. Part A 33, 1608–1619. doi: 10.1080/19440049.2016.1219071
Scudamore, K. A., and Satel, S. (2009). Occurrence of Fusarium mycotoxins in maize imported into the UK, 2004–2007. Food Addit. Contam. Part A 26, 363–371. doi: 10.1080/02652030802406219
Segvic, M., Cvetnic, Z., Pepeljnjak, S., and Kosalec, I. (2009). Co-occurrence of aflatoxins, ochratoxin A, fumonisins, and zearalenone in cereals and feed, determined by competitive direct enzyme-linked immunosorbent assay and thin- layer chromatography. Arch. Ind. Hyg. Toxicol. 60, 427–434. doi: 10.2478/10004-1254-60-2009-1975
Selvaraj, J. N., Wang, Y., Zhou, L., Zhao, Y., Xing, F., Dai, X., et al. (2015). Recent mycotoxin survey data and advanced mycotoxin detection techniques reported from China: A review. Food Addit. Contam. Part A. 32, 440–452. doi: 10.1080/19440049.2015.1010185
Senthilkumar, T., Jayas, D. S., White, N. D. G., Fields, P. G., and Grafenhan, T. (2016). Nearinfrared (NIR) hyperspectral imaging: theory and applications to detect fungal infection andmycotoxin contamination in food products. Indian J. Entomol. 78:91. doi: 10.5958/0974-8172.2016.00029.8
Senthilkumar, R. P., Natarajan, A., and Kavitha, R. (2021). Co-occurrence of mycotoxins in pearl millet. Pharma Innov. J. 10, 253–255.
Serra, R., Bragab, A., and Venancio, A. (2005). Mycotoxin-producing and other fungi isolated from grapes for wine production, with particular emphasis on ochratoxin A. Res. Microbiol. 156, 515–521. doi: 10.1016/j.resmic.2004.12.005
Shetty, P. H., and Bhatt, R. V. (1997). Natural occurrence of fumonisin B1 and its cooccurrence with aflatoxin B1 in Indian sorghum, maize, and poultry feeds. J. Agric. Food Chem. 45, 2170–2173. doi: 10.1021/jf960607s
Shi, H., Schwab, W., Liu, N., and Yu, P. (2019). Major ergot alkaloids in naturally contaminated cool-season barley grain grown under a cold climate condition in western Canada, explored with near-infrared (NIR) and fourier transform mid-infrared (ATRFT/MIR) spectroscopy. Food Control 102, 221–230. doi: 10.1016/j.foodcont.2019.03.025
Shukla, S., Shankar, R., and Singh, S. P. (2014). Food safety regulatory model in India. Food Control 37, 401–413. doi: 10.1016/j.foodcont.2013.08.015
Shukla, R., Singh, P., Prakash, B., and Dubey, N. K. (2012). Antifungal, aflatoxin inhibition and antioxidant activity of Callistemon lanceolatus (Sm.) sweet essential oil and its major component 1,8-cineole against fungal isolates from chickpea. Food Control 25, 27–33. doi: 10.1016/j.foodcont.2011.10.010
Sindhu, S., Chempakam, B., Leela, N. K., and Suseela Bhai, R. (2011). Chemoprevention by essential oil of turmeric leaves (Curcuma longa L.) on the growth of Aspergillus flavus and aflatoxin production. Food Chem. Toxicol. 49, 1188–1192. doi: 10.1016/j.fct.2011.02.014
Singh, J., and Mehta, A. (2020). Rapid and sensitive detection of mycotoxins by advanced and emerging analytical methods: A review. Food Sci. Nutr. 8, 2183–2204. doi: 10.1002/fsn3.1474
Siruguri, V., Kumar, U. P., Raghu, P., Rao, V. V. M., Sesikeran, B., Toteja, G. S., et al. (2012). Aflatoxin contamination in stored rice variety PAU 201 collected from Punjab, India. Ind. J. Med. Res. 136, 89–97.
Smith, C. A., Woloshuk, C. P., Robertson, D., and Payne, G. A. (2007). Silencing of the aflatoxin gene cluster in a diploid strain of Aspergillus flavus is suppressed by ectopic aflR expression. Genetics 176, 2077–2086. doi: 10.1534/genetics.107.073460
Soldatkin, O. O., Stepurska, K., Arkhypova, V., Soldatkin, A., El’Skaya, A., Lagarde, F., et al. (2017). Conductometric enzyme biosensor for patulin determination. Sensors Actuators B Chem. 239, 1010–1015. doi: 10.1016/j.snb.2016.08.121
Soliman, K. M., and Badeaa, R. I. (2002). Effect of oil extracted from some medical plants on different mycotoxigenic fungi. Food Chem. Toxicol. 4, 1669–1675. doi: 10.1016/ s0278-6915(02)00120-5
Sonker, N., Pandey, A. K., and Singh, P. (2014b). Efficiency of Artemisia nilagirica (Clarke) Pamp. essential oil as a mycotoxicant against postharvest mycobiota of table grapes. J. Sci. Food Agric. 95, 1932–1939. doi: 10.1002/jsfa.6901
Sonker, N., Pandey, A. K., Singh, P., and Tripathi, N. N. (2014a). Assessment of Cymbopogon citratus (DC.) Stapf essential oil as herbal preservatives based on antifungal, antiaflatoxin and antiochratoxin activities and in vivo efficacy during storage. J. Food Sci. 79, 628–634. doi: 10.1111/1750-3841.12390
Srianujata, S. (2011). “Regulatory update and control measures for prevention and reduction of mycotoxins contamination in foods and feeds.” in Proceedings of FFTC-KU 2011 Conference: International Seminar ON Risk Assessment and Risk Management of Mycotoxins for Food Safety in Asia. Kasetsart University, Bangkok, Thailand.
Ssepuuya, G., Van Poucke, C., Ediage, E. N., Mulholland, C., Tritscher, A., Verger, P., et al. (2018). Mycotoxin contamination of sorghum and its contribution to human dietary exposure in four sub-Saharan countries. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 35, 1384–1393. doi: 10.1080/19440049.2018.1461253
Sultana, B., Naseer, R., and Nigam, P. (2015). Utilization of agro-wastes to inhibit aflatoxins synthesis by Aspergillus parasiticus: a biotreatment of three cereals for safe long-term storage. Bioresour. Technol. 197, 443–450. doi: 10.1016/j.biortech.2015.08.113
Sulyok, M., Berthiller, F., Krska, R., and Schuhmacher, R. (2006). Development and validation of a liquid chromatography/tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize. Rapid Commun. Mass Spectrom. 20, 2649–2659. doi: 10.1002/rcm.2640
Tanaka, K., Sago, Y., Zheng, Y., Nakagawa, H., and Kushiro, M. (2007). Mycotoxins in rice. Int. J. Food Microbiol. 119, 59–66. doi: 10.1016/j.ijfoodmicro.2007.08.002
Tangni, E. K., Theys, R., Mignolet, E., Maudoux, M., Michelet, J. Y., and Larondelle, Y. (2003). Patulin in domestic and imported apple-based drinks in Belgium: occurrence and exposure assessment. Food Addit. Contam. 20, 482–489. doi: 10.1080/0265203031 000093204
Tebele, S. M., Gbashi, S., Adebo, O., Changwa, R., Naidu, K., and Njobeh, P. B. (2020). Quantification of multimycotoxin in cereals (maize, maize porridge, sorghum and wheat) from Limpopo province of South Africa. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 37, 1922–1938. doi: 10.1080/19440049.2020.1808715
Thanushree, M. P., Sailendri, D., Yoha, K. S., Moses, J. A., and Anandharamakrishnan, C. (2019). Mycotoxin contamination in food: an exposition on spices. Trends Food Sci. Technol. 93, 69–80. doi: 10.1016/j.tifs.2019.08.010
Tola, M., Kebede, B., and Yildiz, F. (2016). Occurrence, importance and control of mycotoxins: A review. Cog. Food Agric. 2:1191103. doi: 10.1080/23311932.2016.1191103
Tölgyesi, Á., Kozma, L., and Sharma, V. K. (2020). Determination of Alternaria toxins in sunflower oil by liquid chromatography isotope dilution tandem mass spectrometry. Molecules 25:1685. doi: 10.3390/molecules25071685
Topi, D., Babic, J., Pavsic-Vrtac, K., Tavcar-Kalcher, G., and Jakovac-Strajn, B. (2021). Incidence of Fusarium mycotoxins in wheat and maize from Albania. Molecules 26:172. doi: 10.3390/molecules26010172
Trombete, F. M., Porto, Y. D., Freitas-Silva, O., Pereira, R. V., Direito, G. M., Saldanha, T., et al. (2017). Efficacy of ozone treatment on mycotoxins and fungal reduction in artificially contaminated soft wheat grains. J. Food Process. Preserv. 41:e12927. doi: 10.1111/jfpp.12927
Tseng, T. C., and Tu, J. C. (1997). Mycoflora and mycotoxins in adzuki and mung beans produced in Ontario, Canada. Microbios 90, 87–95.
Turner, N. W., Bramhmbhatt, H., Szabo-Vezse, M., Poma, A., Coker, R., and Piletsky, S. A. (2015). Analytical methods for determination of mycotoxins: an update (2009-2014). Anal. Chim. Acta 901, 12–33. doi: 10.1016/j.aca.2015.10.013
Van Egmond, H. P., and Jonker, M. A. (2008). “Regulations and limits for mycotoxins in fruits and vegetables” in Mycotoxins in fruits and vegetables. eds. R. Barkai-Golan and N. Paster (San Diego, CA: Elsevier), 45–74.
Velluti, A., Sanchis, V., Ramos, A. J., Egido, J., and Mari, S. (2003). Inhibitory effect of cinnamon, clove, lemongrass, oregano and palmarose essential oils on growth and fumonisin B1 production by Fusarium proliferatum in maize grain. Int. J. Food Microbiol. 89, 145–154. doi: 10.1016/S0168-1605(03)00116-8
Velluti, A., Sanchis, V., Ramos, A. J., Turon, C., and Marín, S. (2004). Impact of essential oils on growth rate, zearalenone and deoxynivalenol production by Fusarium graminearum under different temperature and water activity conditions in maize grain. J. Appl. Microbiol. 96, 716–724. doi: 10.1111/j.1365-2672.2004.02212.x
Vismer, H. F., Shephard, G. S., Rheeder, J. P., van der Westhuizen, L., and Bandyopadhyay, R. (2015). Relative severity of fumonisin contamination of cereal crops in West Africa. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 32, 1952–1958. doi: 10.1080/19440049.2015.1084654
Vismer, H. F., Shephard, G. S., van der Westhuizen, L., Mngqawa, P., Bushula-Njah, V., and Leslie, J. F. (2019). Mycotoxins produced by Fusarium proliferatum and F. pseudonygamai on maize, sorghum and pearl millet grains in vitro. Int. J. Food Microbiol. 296, 31–36. doi: 10.1016/j.ijfoodmicro.2019.02.016
Vithu, P., and Moses, J. A. (2016). Machine vision system for food grain quality evaluation: A review. Trends Food Sci. Technol. 56, 13–20. doi: 10.1016/j.tifs.2016.07.011
Wang, M., Jiang, N., Xian, H., Wei, D., Shi, L., and Feng, X. (2016). A single-step solid phase extraction for the simultaneous determination of 8 mycotoxins in fruits by ultrahigh performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 1429, 22–29. doi: 10.1016/j.chroma.2015.12.004
Wang, B., Mahoney, N. E., Pan, Z., Khir, R., Wu, B., Ma, H., et al. (2016). Effectiveness of pulsed light treatment for degradation and detoxification of aflatoxin B1 and B2 in rough rice and rice bran. Food Control 59, 461–467. doi: 10.1016/j.foodcont.2015.06.030
Wielogorska, E., Ahmed, Y., Meneely, J., Graham, W. G., Elliott, C. T., and Gilmore, B. F. (2019a). A holistic study to understand the detoxification of mycotoxins in maize and impact on its molecular integrity using cold atmospheric plasma treatment. Food Chem. 301:125281. doi: 10.1016/j.foodchem.2019.125281
Wielogorska, E., Mooney, M., Eskola, M., Ezekiel, C. N., Stranska, M., Krska, R., et al. (2019b). Occurrence and human-health impacts of mycotoxins in Somalia. J. Agric. Food Chem. 67, 2052–2060. doi: 10.1021/acs.jafc.8b05141
Wilson, J. P., Jurjevic, Z., Hanna, W. W., Wilson, D. M., Potter, T. L., and Coy, A. E. (2006). Host-specific variation in infection by toxigenic fungi and contamination by mycotoxins in pearl millet and corn. Mycopathologia 161, 101–107. doi: 10.1007/ s11046-005-0170-7
Wolf, K., and Schweigert, J. F. (2018). Mycotoxin analysis: a focus on rapid methods; Partnership for Aflatoxin Control in Africa: Addis Ababa, Ethiopia, 2018. J. Chromatogr. A 1304, 61–68.
Xia, X., Wang, H., Yang, H., Deng, S., Deng, R., Dong, Y., et al. (2018). Dual-terminal stemmed aptamer beacon for label-free detection of aflatoxin B1 in broad bean paste and peanut oil via aggregation-induced emission. J. Agric. Food Chem. 66, 12431–12438. doi: 10.1021/acs.jafc.8b05217
Xie, L., Chen, M., and Ying, Y. (2016). Development of methods for determination of aflatoxins. Crit. Rev. Food Sci. Nutr. 56, 2642–2664. doi: 10.1080/10408398.2014.907234
Xing, F., Hua, H., Selvaraj, J. N., Yuan, Y., Zhao, Y., Zhou, L., et al. (2014). Degradation of fumonisin B1 by cinnamon essential oil. Food Control 38, 37–40. doi: 10.1016/j. foodcont.2013.09.045
Xu, W., Han, X., and Li, F. (2019). Co-occurrence of multi-mycotoxins in wheat grains harvested in Anhui province, China. Food Control 96, 180–185. doi: 10.1016/j. foodcont.2018.09.006
Yamamoto-Ribeiro, M. M., Grespan, R., Kohiyama, C. Y., Ferreira, F. D., Mossini, S. A., Silva, E. L., et al. (2013). Effect of Zingiber officinale essential oil on Fusarium verticillioides and fumonisin production. Food Chem. 141, 3147–3152. doi: 10.1016/j. foodchem.2013.05.144
Yang, Y., Li, G., Wu, D., Liu, J., Li, X., Luo, P., et al. (2020). Recent advances on toxicity and determination methods of mycotoxins in foodstuffs. Trends Food Sci. Technol. 96, 233–252. doi: 10.1016/j.tifs.2019.12.021
Yao, H., Hruska, Z., and DiMavungu, J. D. (2015). Developments in detection and determination of aflatoxins. World Mycotoxin J. 8, 181–191. doi: 10.3920/ WMJ2014.1797
Younis, M., Younis, A., and Xia, X. H. (2020). “Use of biosensors for mycotoxins analysis in food stuff ” in Nanobiosensors: From design to applications. eds. A. Wu and W. S. Khan (Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA), 171–201.
Zhang, L., Dou, X. W., Zhang, C., Logrieco, A. F., and Yang, M. H. (2018). A review of current methods for analysis of mycotoxins in herbal medicines. Toxins 10:65. doi: 10.3390/toxins10020065
Zhang, W., Han, Y., Chen, X., Luo, X., Wang, J., Yue, T., et al. (2017). Surface molecularly imprinted polymer capped Mn-doped ZnS quantum dots as a phosphorescent nanosensor for detecting patulin in apple juice. Food Chem. 232, 145–154. doi: 10.1016/j.foodchem.2017.03.156
Zhang, K., Schaab, M. R., Southwood, G., Tor, E. R., Aston, L. S., Song, W., et al. (2017). A collaborative study: determination of mycotoxins in corn, peanut butter, and wheat flour using stable isotope dilution assay (SIDA) and liquid chromatographytandem mass spectrometry (LC-MS/MS). J. Agric. Food Chem. 65, 7138–7152. doi: 10.1021/acs.jafc.6b04872
Zhang, Z., Tang, X., Wang, D., Zhang, Q., Li, P., and Ding, X. (2015). Rapid on-site sensing aflatoxin B1 in food and feed via a chromatographic time-resolved fluoroimmunoassay. PLoS One 10:e0123266. doi: 10.1371/journal.pone.0145988
Zhu, Y., Xia, X., Deng, S., Yan, B., Dong, Y., Zhang, K., et al. (2019). Label-free fluorescent aptasensing of mycotoxins via aggregation-induced emission dye. Dyes Pigments 170:107572. doi: 10.1016/j.dyepig.2019.107572
Zinedine, A., Brera, C., Elakhdari, S., Catano, C., Debegnach, F., Angelini, S., et al. (2006). Natural occurrence of mycotoxins in cereals and spices commercialized in Morocco. Food Control 17, 868–874. doi: 10.1016/j.foodcont.2005.06.001
Zinedine, A., Soriano, J. M., Juan, C., Mojemmi, B., Moltó, J. C., Bouklouze, A., et al. (2007). Incidence of ochratoxin A in rice and dried fruits from Rabat and Salé area, Morocco. Food Addit. Contam. 24, 285–291. doi: 10.1080/02652030600967230
Zohri, A. A., and Abdel-Gawad, K. M. (1993). Survey of mycoflora and mycotoxins of some dried fruits in Egypt. J. Basic Microbiol. 33:279. doi: 10.1002/jobm.3620330413
Zouaoui, N., Sbaii, N., Bacha, H., and Abid-Essefi, S. (2015). Occurrence of patulin in various fruit juice marketed in Tunisia. Food Control 51, 356–360. doi: 10.1016/j. foodcont.2014.09.048
Zougagh, M., and Ríos, Á. (2008). Supercritical fluid extraction of macrocyclic lactone mycotoxins in maize flour samples for rapid amperometric screening and alternative liquid chromatographic method for confirmation. J. Chromatogr. A 1177, 50–57. doi: 10.1016/j.chroma.2007.11.021