mycotoxins with production, health and reproduction in dairy cattle
An association of mycotoxins with production, health and reproduction in dairy cattle and guidelines for prevention and treatment
The mycotoxicoses which may be most commonly associated with grazing cattle include ergotism, paspalum staggers, fescue toxicity, sweet clover poisoning, facial eczema and slaframine toxicity. These and other mycotoxicoses are important and have been reviewed by Lacey (1991).
This paper will concentrate on those mycotoxins that are of greatest concern for dairy cattle consuming stored feeds. These include aflatoxin, fumitremorgens, and sterigmatocystin, which are primarily produced by Aspergillus molds; deoxynivalenol, zearalenone, T-2, diacetoxyscirpenol (DAS) and fumonisins, which are produced by Fusarium molds; and ochratoxin, PR toxin and roquefortine, which are primarily produced by Penicillium molds. Several other mycotoxins, produced by these and other molds, are known to be prevalent at times, including derivatives of those listed. It is probable that a lack of observation and simple analytical techniques have prevented us from more fully understanding the prevalence of these mycotoxins and their impact on animal production.
Mold growth and mycotoxin formation
Molds occur universally in a variety of feedstuffs, including roughages and concentrates and can produce mycotoxins under certain conditions.
Molds can grow and mycotoxins can be produced pre-harvest or postharvest, during storage, processing, or feeding. Mycotoxin production is often related to extremes in weather conditions (causing plant stress or excess hydration of stored feedstuffs), to inadequate storage practices, to low feedstuff quality and to faulty feeding conditions.
Conditions for mold growth and mycotoxin formation are dependent on the specific mold but include the presence of fungal spores, an organic substrate and the proper levels of moisture, oxygen, temperature, and acidity (Moss, 1991). Temperatures may range from -5 to 60 °C. Water activity must generally be above 0.7 aw (ratio of the vapor pressure of the product to that of pure water or equilibrium relative humidity as a percentage).
Mold can begin growing when moisture exceeds about 12%. Higher levels of moisture will support mold growth up to the point where water excludes adequate oxygen. High levels of carbon dioxide can prevent mold growth even when oxygen is at levels high enough to support mold growth. Oxygen as low as 0.5% can support mold growth; thus there can be pockets of adequate oxygen within silage and stored high moisture grain storage, especially near the feed surfaces.
A fairly wide range of pH levels will support mold growth, although they do not grow well at extremely low or high pH levels.While silage pH is generally low enough to prevent most mold growth, yeasts are active at a lower pH and their activity can raise the pH to a point conducive to mold growth.
The Aspergillus species grow at lower water activities and at higher temperatures than do the Fusarium species, which generally require higher water activities but are able to grow atmuch lower temperatures. Aspergillus flavus and aflatoxin in corn are favored by the heat and drought stress associated with warmer climates.
Aflatoxin seems to be enhanced by insect damage before and after harvest. Penicillium species grow at relatively low water activities and low temperatures and are fairly widespread in occurrence. Since both Aspergillus and Penicillium grow at low water activities, they are considered the more likely storage fungi, with Aspergillus more likely in warm climates and Fusarium and Penicillium more likely in cooler climates.
The Fusarium species are generally considered to be field fungi and more likely to proliferate prior to storage. Fusarium commonly affects corn, causing ear and stalk rot, and small grains, causing field diseases such as head blight (scab). These field diseases are characterized by yield loss, quality loss and mycotoxin contamination.
In wheat, excess moisture at flowering and afterward is associated with increased incidence of mycotoxin formation. In corn, Fusarium diseases are more commonly associated with insect damage, warm conditions at silking and wet conditions late in the growing season. Joffe (1986) suggests that the toxic principle in the soil spreads to the plant, first affecting the vegetative parts and then the grain. The grain provides a favorable substrate for toxin accumulation. Trenholm et al. (1988) suggest that plowing in plant debris and crop residue left on the field after harvest may reduce fungal disease problems.
It should be noted that the conditions most suitable for mold growth are not necessarily the optimum conditions for mycotoxin formation. For example, the Fusarium molds associated with alimentary toxic aleukia have been reported to grow prolifically at temperatures of 25 to 30 °C without producingmuchmycotoxin but at near freezing temperatures large quantities of mycotoxins are produced without much mold growth (Joffe, 1986).
Mycotoxin occurrence
The warm, humid climate of the southern US results in a considerably higher incidence of aflatoxin in feeds. From 1975 to 1980, 34% of corn grain in North Carolina contained more than 20 ppb aflatoxin. Corn grain and peanut meal have been the primary sources of aflatoxin contamination in North Carolina. Cottonseed has seldom been a problem source of aflatoxin for dairymen in North Carolina. Corn samples from the midwestern US representing the 1988 season (severe drought) showed 8% with aflatoxin levels above 10 ppb, 3% positive for zearalenone above 1 ppm, 3% positive for deoxynivalenol above 1 ppm and 7% positive for T-2 above 500 ppb (Russel et al., 1991).
Mycotoxin analysis results from feed samples submitted by North Carolina farmers during a nine-year period and representing more than 2400 samples were summarized (Whitlow et al., 1998). Percentage of corn silage and corn grain samples testing positive were for aflatoxin (>10 ppb), 8 and 9%; deoxynivalenol (>500 ppb), 51 and 52%; zearalenone (300 ppb), 17 and 3%; T-2 (>200), 5 and 4% and fumonisin B1 (>1 ppm), 37 and 60%, respectively. Occurrence was highly variable by year.
Mycotoxin effects
Mycotoxins can increase disease incidence and reduce production efficiency in cattle. They exert their effects through three primary mechanisms:
1. alteration in nutrient content, absorption and metabolism
2. changes in the endocrine and neuroendocrine function
3. suppression of the immune system (CAST, 1989)
The resulting nonspecific symptomsmay therefore be perplexing and make diagnosis difficult. Hesseltine (1986b) and Schilfer (1990) discussed some of the problems encountered in diagnosing a mycotoxicosis. They include:
1. a lack of research reports especially concerning some mycotoxins
2. symptoms that are not specific or unique for the mycotoxin
3. interaction of mycotoxins with othermycotoxins or other stress factors
4. interaction of mycotoxins with immune suppression and thus infectious diseases
5. lack of feed samples or samples improperly collected
6. analysis which is complex and expensive
Our experience suggests that while a definitive diagnosis cannot be made directly from symptoms, specific tissue damage, or even feed analyses, experience with mycotoxin-affected herds greatly increases the probability of recognizing the problem. The following guidelines may be helpful in dealing with a possible mycotoxicosis:
1. Mycotoxins should be considered as a possible primary factor resulting in production losses and increased incidence of disease.
2. Documented symptoms in ruminants or other species can be utilized as a general guide to symptoms observed in the field; however there is a lack of research data, and field observations may differ from those seen in controlled research studies.
3. Systemic effects as well as specific damage to target tissues can be used as a guide to possible causes.
4. Postmortem examinations may indicate no more than gut irritation, edema or generalized tissue inflammation.
5. Ruling out other possible causes such as infectious agents or other toxins is essential.
6. All feeds should be analyzed for common mycotoxins.
7. Responses to simple treatments such as dilution or removal of the contaminated feed are helpful.
8. Diagnosis may be impossible because the clinical situation may be complex and complicated due to interactions with other agents.
Dairy herds experiencing a mycotoxicosis severe enough to reduce milk production will usually display other symptoms. Often there is intermittent diarrhea, sometimes with bloody or dark manure. Cows may not respond well to typical veterinary therapy. Symptoms may be nonspecific and wide ranging and may include reduced feed intake, feed refusal, ill thrift, rough hair coat, undernourished appearance, subnormal production, increased abortions or embryonic mortalities, silent heats, irregular estrus cycles, expression of estrus in pregnant cows and decreased conception rates. Fresh cows perform poorly and generally have an increased incidence of disease, particularly those that are most opportunistic in a dairy herd. There may be a higher incidence of displaced abomasum, ketosis, retained placenta, metritis, mastitis and fatty livers. There may only be a few or many of these symptoms evident.
AFLATOXIN
Aflatoxin, produced primarily by Aspergillus flavus, is amycotoxin of major concern because it is carcinogenic and is commonly found in warmclimates such as the southern US. Major efforts are directed at eliminating food residues. The FDA limits aflatoxin in corn grain according to its intended use: not more than 200 ppb for breeding cattle, 300 ppb for finishing beef cattle or 20 ppb for lactating dairy cattle.
Aflatoxin is excreted into milk in the form of aflatoxin M1 with residues approximately equal to 1.7% of the dietary aflatoxin level (Van Egmond, 1989). The FDA limits aflatoxin M1 in milk to no more than 0.5 ppb. Since aflatoxin residues can be found in tissues, beef cattle should not be fed aflatoxin contaminated diets for three weeks prior to slaughter. Regulatory pressures and a widespread awareness have helped minimize aflatoxin problems. The GAO (1991) concluded that industry, federal and state programs are effective in detecting and controlling aflatoxin and that it is doubtful that additional programs or limits would reduce the risk of aflatoxin in the food supply. Thus, current surveillance programs aimed at reducing food residues make it very unlikely for aflatoxin to have significant production or health effects on dairy herds.
Aflatoxin can reduce performance and impair health, but this occurs generally at dietary levels greater than the 25 to 50 ppb that can cause illegal milk residues. Although no level of aflatoxin is considered safe, the degree of toxicity is related to level of toxin, duration of feeding, and the amount of other stresses affecting the animal. Levels of 300 to 700 ppb are considered toxic for beef cattle, depending on criteria for toxicity, and other factors affecting toxicity (CAST, 1989). Garrett et al. (1968) showed that with beef cattle gain and intake were affected at 700 ppb, but not at 300 ppb aflatoxin; however, levels of no effect cannot be determined from data with such few animals.
Trends in the data, especially for increased liver weights, would indicate potential toxicity at levels as low as 100 ppb. Guthrie (1979) showed a decline in reproductive efficiency when lactating dairy cattle in a field situation were consuming 120 ppb aflatoxin and an increase in milk production of over 25% when cows were changed to an aflatoxin-free diet. Patterson and Anderson (1982) and Marsi et al. (1969) also suggest that 100 ppb may reduce milk production. Applebaum et al. (1982) showed that impure aflatoxin produced by culture reduced production, but equal amounts of pure aflatoxin did not. Several studies suggest that naturally contaminated feeds are more toxic than would be expected from the concentrations of assayed mycotoxins, suggesting the presence of unidentified toxins.
FUMONISIN
Fumonisin B1 was isolated by Gelderblom et al. (1988) and shown to be a cancer promoter. Fumonisin B1 has been shown to cause leukoencephalomalacia in horses (Marasas et al., 1988), pulmonary edema in pigs (Harrison et al., 1990) and hepatoxicity in rats (Gelderblom et al., 1991). A USDA APHIS (1995) survey found an average of 6.9% of 1995 corn samples from Missouri, Iowa and Illinois to contain more than 5 ppm fumonisin B1.
While fumonisin B1 is thought to bemuch less potentin ruminants than monogastrics, work by Kriek et al. (1981) suggested that fumonisin was toxic to sheep. Osweiler et al. (1993) demonstrated that fumonisin B1 in large amounts (148 ppm) can cause mild liver damage in cattle even when fed for a short term (31 days), but had no effect on feed intake or weight gain.Whitlow (1999, unpublished) has demonstrated that fumonisin B1 is also toxic to dairy cattle. Fed for approximately seven days prior to freshening and for 70 days thereafter, dietary fumonisin B1 at 100 ppm significantly and dramatically reduced milk production (7 kg/cow/day) and affected serum enzymes levels indicative of liver disease.
These results strongly suggest that fumonisin B1 is toxic to dairy cattle and that it is less toxic to beef cattle, or perhaps fumonisin B1 interacts with other factors to produce greatly different effects in beef and dairy cattle under different conditions.
Fumonisin B1 carryover from feed to milk is thought to be negligible. Richard et al. (1996) fed fumonisin B1 (about 75 ppm) to dairy cows with no fumonisin B1 or B2 detectable in milk (detection limit of 5 ng/ml). Scott et al. (1994) have confirmed this observation.
DEOXYNIVALENOL
Deoxynivalenol (DON) is the proper name for a commonly detected Fusarium-produced mycotoxin often referred to as vomitoxin. Two independent midwestern US studies (Vesonder et al., 1978 and Coˆte´ et al., 1984) showed deoxynivalenol to be the primary mycotoxin associated with problems in pigs including feed refusals, diarrhea, emesis, reproductive failure and deaths. In cattle, deoxynivalenol has been associated with reduced feed intake (Trenholm et al., 1985) and lower milk production (Whitlow et al., 1991). Clinical data from 300 herds representing about 40,000 cow records showed that deoxynivalenol was associated with a loss in milk production but did not establish a cause and effect relationship (Whitlow et al., 1991). Deoxynivalenol may simply be a marker for problem feeds. Field observations by others help substantiate these observations (Gotlieb, 1997; Seglar, 1997).
Charmley et al. (1993) demonstrated a 13% (2.85 kg) numerical decrease in 4% fat corrected milk production (statistics not available) utilizing 18 mid-lactation dairy cows (average 19.5 kg milk) consuming diets shown to contain no common mycotoxins other than deoxynivalenol, which was at levels of 2.7 to 6.4 ppm in treatment diets.While the decrease in actual milk production (1.35 kg) was not statistically significant, the decrease in fat test (3.92% vs. 3.04%) was significant.
Noller et al. (1979) utilized 54 lactating dairy cows in a 21-day feeding experiment using corn grain contaminated with Gibberella zeae and containing 500 ppb zearalenone (ZEN). Deoxynivalenol was probably present, but it was not analyzed directly. Grain harvested earlier from the same field was contaminated with 12-13 ppm DON. Neither dry matter intake nor milk production (average 22.9 kg) were affected by additions of this grain to the diet. However, compared with controls, cows that received this grain at either 10% (about 1.25 ppm DON and 50 ppb ZEN) or 20% (about 2.50 ppm DON and 100 ppb ZEN) of the diet gained significantly less weight during the study (5.8 or 8.1 kg less weight gain for cows consuming the 10% or 20% diets over 21 days, respectively).
DiCostanzo et al. (1995a) cites results by Ingalls (1994) where lactating dairy cows were fed 0, 3.6, 10.9 and 14.6 ppm DON for 21 days, apparently without effect on feed intake or milk production, which averaged about 30 kg daily.
Beef cattle and sheep appear to tolerate relatively large amounts of deoxynivalenol without obvious deleterious effects. Reports from Nebraska indicated similar feed intakes, average daily gains and feed efficiencies when sheep (8.5 ppp dietary DON) or cattle (1 ppm dietary DON) consuming deoxynivalenol-contaminated diets were compared with those consuming diets containing no detectable deoxynivalenol (DeHaan et al., 1984).
Nelson et al. (1984) fed feedlot steers and heifers diets containing 0.2, 2.3 or 10 ppm DON for 126 days. The low DON diet was corn-based while the other two diets contained wheat. Results reported for the low, medium and high DON diets were similar for dry matter (DM) intake (9.4, 8.7 and 7.8 kg/day), average daily gain (1.54, 1.64 and 1.34 kg/day) and feed efficiency (6.2, 5.6 and 5.7 kg DM intake/kg gain). Results for carcass characteristics, serum biochemistry and tissue histology were similar across treatments. DiCostanzo et al. (1995a and 1995b) indicated that feeding up to 18 ppm dietary DON did not affect intake, daily gain, feed efficiency or carcass characteristics of 415 kg steers fed for 166 days.
Other recent feeding experiments with beef cattle suggest that they can tolerate large concentrations of deoxynivalenol in a feedlot situation without affecting dry matter intake, average daily gain or feed efficiency (Boland et al., 1994; Windels et al., 1995).
These data suggest that cattle are relatively tolerant of deoxynivalenol. While not compared directly, it appears that beef cattle and sheep may be less sensitive to deoxynivalenol than are dairy cattle. Differences could be related to level of production stress, since mid-lactation, low-producing dairy cattle also appear to be more tolerant of deoxynivalenol than are high-producing dairy cattle in early lactation. Mycotoxins may interact with immune suppression in early lactation to produce more severe effects than would otherwise be expected. Heat or other environmental stresses may be involved. Thus, the early-lactation, high-producing cows which experience greater stress, lower immunity, marginal nutrient deficiencies and a faster rumen turnover (less mycotoxin degradation in the rumen) may be more vulnerable to mycotoxin effects.
Deoxynivalenol is but one causative agent that may be present. Deoxynivalenol may serve as a marker for feed exposed to a situation conducive to mold growth andmycotoxin formation, and thus the possible presence of other mycotoxins or factors more toxic than deoxynivalenol itself. The differences in response to deoxynivalenol may be due to other mycotoxins. Experiments with beef cattle have generally utilized DON-contaminated corn or barley. Deoxynivalenol provided by contamination of a different feed source, such as silage, could result in interactions of differentmycotoxins.Mycotoxin interactions are discussed more fully in the section entitled ‘Safe levels of mycotoxins’.
T-2 TOXIN
T-2 toxin, a Fusarium-produced mycotoxin, has been associated with gastroenteritis, intestinal hemorrhages (Petrie et al., 1977 and Mirocha et al., 1976) and death (Hsu et al., 1972, Kosuri et al., 1970). Weaver et al. (1980) demonstrated that T-2 was associated with feed refusal and gastrointestinal lesions in a cow, but failed to show a hemorrhagic syndrome. Serum immunoglobulins and certain complement proteins were lowered in calves receiving T-2 toxin (Mann et al., 1983). Other data demonstrated a reduction in white blood cell and neutrophil counts in calves (Gentry et al., 1984). A calf intubated with T-2 developed severe depression, hindquarter ataxia, knuckling of the rear feet, listlessness and anorexia (Weaver et al., 1980).
While data with cattle are limited, the toxicity of T-2 toxin in laboratory animals is well-documented (Wannemacher et al., 1991). Our experience suggests that T-2 is a severe gastrointestinal irritant, which can cause hemorrhage and necrosis of the intestinal tract. Diarrhea is usually present but may not be hemorrhagic. With high levels of T-2, there can be congestion and irritation to the liver, lungs and heart.
Two dairy herds were observed to receive T-2 contaminated feed from the same supplier and on similar dates. Early lactation cows were more severely affected, showing a lack of appetite, severe and prolonged weight loss, low peak milk production, higher levels of morbidity and death. In another field case, T-2 in corn produced on the farm resulted in approximately 350 ppb in the diet. Cows exhibited diarrhea, which moved in a wave over time through a dairy herd of about 150 Jersey cows. Milk production was erratic for two to three days and then dropped by 15%. The addition of a clay product to the diet appeared to restore production to previous levels after about three weeks. Removal of the clay resulted in an immediate loss in milk production and the clay was again fed, with a positive response.
ZEARALENONE
Zearalenone is a Fusarium-produced mycotoxin that elicits an estrogenic response in monogastrics (Sundlof and Strickland, 1986). However, zearalenone is rapidly converted to a- and ß-zearalenol in rumen cultures (Kiessling et al., 1984) and has been of less toxicity to ruminants. Ruminal degradation of zearalenone was found to be about 30% in 48 hours (Kellela and Vasenius, 1982). A controlled study with cows fed up to 22 ppm ZEN showed no obvious effects except that corpora lutea were smaller in treated cows (Weaver et al., 1986b). In a similar study with heifers receiving about 13 ppm ZEN, conception rate was depressed about 25%. Otherwise, no obvious effects were noted (Weaver et al., 1986a).
Several case reports have related zearalenone to an estrogenic response in ruminants (Khamis et al., 1986; Mirocha et al., 1968; and Roine et al., 1971). Large doses are associated with abortions in cattle (Kellela and Ettala, 1984; Mirocha et al., 1974). Mirocha et al. (1968) isolated zearalenone from hay associated with infertility in dairy cattle. Other cattle responses may include vaginitis, vaginal secretions, poor reproductive performance and mammary gland enlargement of virgin heifers. In a field study (Coppock et al., 1990), diets with about 750 ppb ZEN and 500 ppb DON resulted in poor consumption, depressed milk production, diarrhea and total reproductive failure.
New Zealand workers (Towers et al., 1995a; 1995b; Sprosen and Towers, 1995; Smith et al., 1995) have related urinary zearalenone and zearalenone metabolites (zearalenone, zearalanone, a- and ß-zearalenol and a- andß-zearalanol) which they refer to as ‘zearalenone’ to intake of ‘zearalenone’ and to reproductive disorders in sheep and dairy cattle. In sheep,‘zearalenone’ was related to lower conception, reduced ovulation, and increased twinning rates. With dairy cattle, herds with low fertility were found to have higher levels of blood and urinary ‘zearalenone’ and consumed pastures containing higher levels of ‘zearalenone’. In addition, within herds, individual cows were examined by palpation and those that were determined to be cycling had lower blood ‘zearalenone’ levels than did cows that were not cycling. Differences in ‘zearalenone’ levels were attributed to selective grazing behavior. The reproductive problems in dairy cattle were noted with ‘zearalenone’ concentrations of about 400 ppb in the pasture samples.
Our observations suggest that zearalenone may be associated with poor conception, early embryonic mortality and increased reproductive tract infections. In most cases, cows have appeared well nourished with average body condition scores but poor reproductive performance. The differences may be attributed to the presence of other mycotoxins or interaction with other factors.
OTHER MYCOTOXINS
Many other mycotoxins may affect ruminants but are thought to occur less frequently or be less potent.
Fumitremorgens such as fumigaclavine A and B are produced by Aspergillus fumigatus and are thought to be common in silages of the southeastern US. They can cause anorexia, diarrhea, ill thrift and irritability (Cole et al., 1977).
Sterigmatocystin is primarily produced by Aspergillus versicolor and has been observed as a primary mycotoxin produced by Aspergillus on cereal grains in western Canada (Mills and Abramson, 1986).While it is thought to be infrequent at toxic levels in the US, it was detected in a grain mixture and associated with bloody diarrhea and cow deaths in a field case in Tennessee (Vesonder and Horn, 1985).
Diacetoxyscirpenol is a Fusarium-produced mycotoxin. It may occur along with T-2 toxin and causes similar symptoms.
Ochratoxin, produced primarily by a Penicillium mold but also by certain Aspergillus molds, has been reported to affect cattle (Vough and Glick, 1993), but it is rapidly degraded in the rumen and thus thought to be of little consequence unless consumed by young pre-ruminant calves (Sreemannarayana et al., 1988). However, high-concentrate diets reduce ochratoxin degradation in the rumen.
Patulin, a Penicillium-produced mycotoxin associated with aerobic deterioration of silage, has been incriminated as a possible toxin in Europe and New Zealand (Lacey, 1991).
PR toxin, produced by Penicillium roquefortii, has been found in silage and was the suspected vector in a case study with symptoms of abortion and retained placenta (Still et al., 1972).
Roquefortine, produced by Penicillium roquefortii, is a tremorgen that has been found in silage.
Other mycotoxins such as rubratoxin, citrinin, cyclopiazonic acid and ergotoxins may be of some importance. Many other mycotoxins are possible.
Mycotoxin testing
To determine toxicity, feeds should be analyzed for mycotoxins and not just mold content; however the type of mold present may suggest the mycotoxins most likely to be present. The amount or presence of mold or mold spore count is not very indicative of mycotoxin content (Wyatt, 1991). Molds may be present which do not produce, or are not currently producing, mycotoxins. A mold may have produced mycotoxins and is no longer viable resulting in mycotoxin levels without the obvious presence of mold. It is possible that opinions have been formed about the toxicity of mycotoxins based on the presence of mold, which might suggest a low or erratic toxicity.
Analytical techniques formycotoxins are improving (Chu, 1992). Several commercial laboratories are available and provide screens for a large array of mycotoxins. Cost of analyses has been a constraint but can be insignificant compared with the economic consequences of production and health losses related to mycotoxin contamination. Newer immunoassays have reduced analytical costs.
Collection of representative feed samples is a problem primarily because molds can produce very large amounts of mycotoxins in small areas making the mycotoxin level highly variable within the lot of feed. Sampling of horizontal silos show mycotoxins to be highly variable throughout the silage; however, the silo face appears to have higher and more consistent levels. Because mycotoxins can form in the collected sample, it should be preserved and delivered to the lab quickly. Samples can be dried, frozen or treated with a mold inhibitor before shipping.
Safe levels of myotoxins
Guidelines for acceptable levels of mycotoxins should be conservatively low due to non-uniformdistribution, uncertainties in sampling and analysis, the potential for more than one source in the diet and the limited amount of research. All these factors make it impossible to declare levels of safety.
Hamilton (1984) and Schaeffer and Hamilton (1991) have reviewed the topic of safe mycotoxin levels. They conclude that epidemiological studies coupled with laboratory studies to elaborate the underlying principles may be the best approach to determining safe levels. They state that any level of mycotoxin carries a risk of loss with it and that it is impossible to define a safe level under laboratory conditions that will be accurate under field conditions. This is primarily because of three reasons:
1. Difficulties in conceptualizing and executing experiments to investigate multiple interacting factors simultaneously;
2. The unappreciated fact that the frequency and level of contamination with aflatoxin and other mycotoxins varies unpredictably under field conditions;
3. Animal facilities currently available to investigators do not permit experiments under controlled conditions with the number of animals commonly at risk under field conditions.
Establishing usable or tolerable levels of mycotoxins may be acceptable when all concerned parties are aware of levels and the risks associated.
Interactions with other factors make recommendations difficult. Lillehoj and Ceigler (1975) give an example where penicillic acid and citrinin were innocuous when administered alone but were 100% lethal when given in combination. Fumonisin at 100 ppm has been shown to reduce milk production in dairy cattle (Whitlow, 1999, unpublished), but did not affect average daily gain in beef cattle fed 148 ppm (Osweiler et al., 1993). Aflatoxin produced from culture was more toxic to dairy cattle than pure aflatoxin added to diets (Applebaum et al., 1982).
In pigs, Foster et al. (1986) demonstrated that pure deoxynivalenol added to diets was less toxic than diets with similar concentrations of deoxynivalenol supplied from naturally contaminated feeds. Smith and MacDonald (1991) have suggested that fusaric acid may occur along with deoxynivalenol to produce more severe symptoms. Many such interactions are possible since Fusarium molds produce many mycotoxins and it is well documented that several mycotoxins may be found in the same feed (Hagler et al., 1984). Abbas et al. (1989) demonstrated that Fusarium species isolated from Minnesota corn produces an array of mycotoxins. Scott (1990) states that screening methods are needed for the Fusarium-produced mycotoxins and that one approach is to test for deoxynivalenol, diacetoxyscirpenol, T-2 and nivalenol, because other Fusarium mycotoxins seldom occur without one of these four also present. Feeds could then be further tested for other mycotoxins.
There are distinct species differences in tolerance to mycotoxins. Cattle are more tolerant to most mycotoxins than many other animals, probably due to some mycotoxin degradation in the rumen (Kiessling et al., 1984). The rat is much more sensitive to both aflatoxin and T-2 than is the mouse (Wannemacher et al., 1991). Other animal factors include sex, age, environmental and production stress. Certainly duration of exposure is important. The known dietary factors that interact with mycotoxins include most nutrients for which rations are formulated including, fat, protein, fiber, vitamins and minerals. Dietary pellet binders (clay) adsorb some mycotoxins reducing exposure of the animal. Thus, many factors and interactions make it difficult to relate field observations to those from controlled research.
Prevention and treatment
Prevention of mycotoxin formation is essential since there are few ways to completely overcome problems once mycotoxins are present. Prevention of mycotoxins may begin with selection of crop varieties that are more resistant to fungal foliar diseases along with use of agronomic practices that may reduce fungal infection of the crop. Prevention of mycotoxins in silage includes following accepted silage-making practices aimed at enhancing proper fermentation and eliminating oxygen.
Silages should be harvested at the correct moisture content, the silo filled rapidly, the silage packed tightly and the silo sealed completely. Silo size should be matched to herd size to ensure daily removal of silage at a rate faster than deterioration (4-6 inches daily, depending on weather). The face of horizontal silos should be cut cleanly while avoiding loosening more silage than is to be fed. Secondary fermentation can occur very rapidly after loosened silage is exposed to the air. Therefore, silage should be fed directly after removal from the silo and feed bunks should be cleaned regularly. Care should be taken to ensure that high-moisture grains are stored at proper moisture contents and in a well-maintained structure. Grains or other dry feed such as hay should be stored at a moisture content below which molds do not readily grow (<14%). Aeration of grain bins is important to reduce moisture migration and to keep the feedstuff dry.
Some additives may be beneficial in reducing mycotoxins because they are effective in reducing mold growth. Ammonia, propionic acid and microbial or enzymatic silage additives have all shown effectiveness as mold inhibitors. It seems reasonable that additives that enhance fermentation may be added at ensiling, while those which inhibit mold growth may be added as surface treatments when capping off the silo or daily after silage feed-out to reduce molding of the exposed silage surface.
If unacceptably high levels of mycotoxins occur, dilution or removal of the contaminated feed is preferable; however, it is usually impossible to completely replace major forage ingredients. Ammoniation of grains can destroy some mycotoxins but there is no practical method to detoxify affected forages already in storage. Increasing nutrients such as protein, energy and antioxidant nutrients may be advisable (Brucato et al., 1986; Chandler, 1992). In some situations, poultry respond to water soluble vitamins. Acidic diets may exacerbate effects of mycotoxins. Additional research on treatments is needed.
Adsorbent materials are not approved by the FDA for the prevention or treatment of mycotoxicoses. However, favorable research results have been seen when adsorbent materials such as clays (bentonites) are added to mycotoxin-contaminated diets of rats, poultry, swine and cattle (Diaz et al., 1998; Galey et al., 1987; Harvey et al., 1988; Lindemann and Blodgett, 1991; Scheideler, 1990; Hayes, 1990; Smith, 1980; 1984).
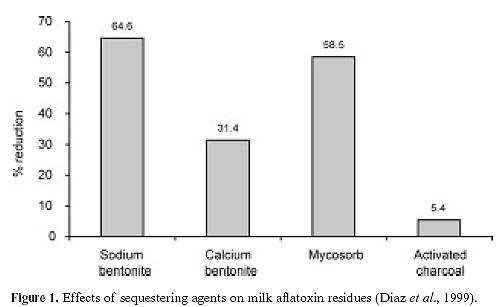
In most cases, clay was added to the diet at about 1%. Considerable data are also available for other absorbent materials such as charcoal, fiber, and yeast cell components. An esterified glucomannan product (Mycosorb, Alltech, Inc.) was shown to reduce milk aflatoxin concentrations by 58% in dairy cows consuming aflatoxin-contaminated diets when included at 0.05% of the diet dry matter (Diaz et al., 1999; Figure 1). The reduction of milk aflatoxin was similar to that seen for a sodium bentonite product included in the diet at 1.1% of the dry matter. The reduction in milk aflatoxin may be a good indicator of strong binding with dietary aflatoxin, reducing aflatoxin absorption through the intestine of the cow.
Areas of needed information
The Council for Agricultural Science and Technology published a list of major needs for research (CAST, 1989). Included in their list were surveillance of feeds for mycotoxin presence and quantity, assessment of control methods, development of resistant plants, improvement of sampling and analysis, improved understanding of effects on animals (particularly on immunosuppression), toxicological evaluation of newly discovered mycotoxins and assessment of economic effects.
References
Abbas, H.K., C.J. Mirocha, T.Kommedahl, R.F. Vesonder and P. Golinski. 1989. Production of trichothecenes and non-trichothecene mycotoxins by Fusarium species isolated from maize in Minnesota. Mycopathologia. 108:55.
Applebaum, R.S., R.E. Brackett, D.W. Wiseman and E.L. Marth. 1982. Responses of dairy cows to dietary aflatoxin: feed intake and yield, toxin content, and quality of milk of cows treated with pure and impure aflatoxin. J. Dairy Sci. 65:1503.
Boland, E.W., V.L. Anderson, H.H. Casper, P.T. Berg andD.V. Dhuyvetter. 1994. The effects of increasing vomitoxin (DON) levels from scab infested barley fed to feedlot steers. Beef Prod. Field Day. Carrington Res. Ext. Ctr. Livestock Unit. North Dakota State University Ag. Expt. Sta. 17:34. (As quoted by DiCostanzo et al., 1995a).
Brucato, M., S. F. Sundlof, J. U. Bell and G. T. Edds. 1986. Aflatoxin B1 toxicosis in dairy calves pretreated with selenium-vitamin E. Am. J. Vet. Res. 47:179.
Chandler, Paul. 1992. Selenium, vitamin E indicate oxidative stress in dairy cattle. Feedstuffs 64(44):10.
Charmley, E., H.L. Trenholm, B.K. Thompson, D. Vudathala, J.W.G. Nicholson, D.B. Prelusky and L.L. Charmley. 1993. Influence of level of deoxynivalenol in the diet of dairy cows on feed intake, milk production and its composition. J. Dairy Sci. 76:3580.
Chu, F.S. 1992. Recent progress on analytical techniques for mycotoxins in feedstuffs. J. Anim. Sci. 70:3950.
Cole, R.J., J.W. Kirksey, J.W.Dorner,D.M.Wilson, J.C. Johnson, Jr., A.N. Johnson, D.M. Bedell, J.P. Springer, K.K. Chexal, J.C. Clardy and R.H. Cox. 1977. Mycotoxins produced by Aspergillus fumigatus species isolated from moldy silage. J. Agric. Food Chem. 25:826.
Coppock, R.W., M.S. Mostrom, C.G. Sparling, B. Jacobsen and S.C. Ross. 1990. Apparent zearalenone intoxication in a dairy herd from feeding spoiled acid-treated corn. Vet. Hum. Toxicol. 32:246.
Coˆte´, L.M., J.D. Reynolds, R.F. Vesonder,W.B. Buck, S.P. Swanson, R.T. Coffey and D.C. Brown. 1984. Survey of vomitoxin-contaminated feed grains in Midwestern United States and associated health problems in swine. J. Am. Vet. Med. Assoc. 184:189.
Council for Agricultural Science and Technology (CAST). 1989. Mycotoxins: Economic and Health Risks. Task Force Report No. 116. Ames, Iowa.
DeHaan, K., R. Stock, D. Brink, T. Klopkenstein and N. Schneider. 1984. Scabby wheat influence on performance. Nebraska Beef Cattle Report. MP-47:6.
Diaz, D.E., J.T. Blackwelder,W.M. Hagler, Jr., B.A. Hopkins, F.T. Jones, K.L. Anderson and L.W.Whitlow. 1998. The potential of dietary clay products to reduce aflatoxin transmission to milk of dairy cows. J. Dairy Sci. 80(Suppl. 1):261.
Diaz, D.E.,W.M. Hagler, Jr., B.A. Hopkins, J.A. Eve and L.W.Whitlow. 1999. The potential for dietary sequestering agents to reduce the transmission of dietary aflatoxin to milk of dairy cows and to bind aflatoxin in vitro. J. Dairy Sci. (Abstract) Southern Branch, American Dairy Science Association, February 1, 1999.
DiCostanzo, A., L. Johnston, H.Windels and M. Murphy. 1995a. A review of the effects of molds and mycotoxins in ruminants. Professional Animal Scientist 12:138.
DiCostanzo, A., J.C. Meiske, M. Murphy, R. Epley, L. Felice, L. Johnson, and H. Chester-Jones. 1995b. Effects of feeding vomitoxin-contaminated barley on performance of feedlot steers. Minnesota Cattle Feeders Rep. B-418, St. Paul, MN. (As quoted by DiCostanzo et al., 1995a).
Foster, B.C., H.L. Trenholm, D.W. Friend, B.K. Thompson and K.E. Hartin. 1986. Evaluation of different sources of deoxynivalenol (vomitoxin) fed to swine. Can. J. Anim. Sci. 66:1149.
Galey, F.D., R.J. Lambert, M. Busse and W.B. Buck. 1987. Therapeutic efficacy of superactive charcoal in rats exposed to oral lethal doses of T-2 toxin. Toxicol. 25:493.
GAO. 1991. Food safety and quality. Existing detection and control programs minimize aflatoxin. Report RCED-91-109.
Garrett,W.N., H. Heitman, Jr. and A.N. Booth. 1968. Aflatoxin toxicity in beef cattle. Proc Soc. Exp. Biol. Med. 127:188.
Gelderblom, W.C.A., K. Jaskiewicz, W.F.O. Marasas, P.G. Thiel, R.M. Horak, R. Vleggaar and N.P.J. Kriek. 1988. Fumonisins: Novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl. Environ. Microbiol. 54:1806.
Gelderblom,W.C.A., N.P.J. Kreik,W.F.O. Marasas and P.G. Thiel. 1991. Toxicity and carcinogenicity of the Fusarium moniliforme metabolite, fumonisin B1, in rats. Carcinogenesis 12:1247.
Gentry, P.A., M.L. Ross and P.K.C. Chan. 1984. Effect of T-2 toxin on bovine hematological and serum enzyme parameters. Vet. Hum. Toxicol. 26:24.
Gotlieb, A. 1997. Causes of mycotoxins in silages. In: Silage: Field to Feedbunk, NRAES-99, Northeast Regional Agricultural Engineering Service, Ithaca, NY. pp. 213.
Guthrie, L.D. 1979. Effects of aflatoxin in corn on production and reproduction in dairy cattle. J. Dairy Sci. 62(Suppl. 1):134.
Hagler, W.M., K. Tyczkowska and P.B. Hamilton. 1984. Simultaneous occurrence of deoxynivalenol, zearalenone and aflatoxin in 1982 scabby wheat from the Midwestern United States. Appl. Environ. Microbiol. 47:151.
Hamilton, P.B. 1984. Determining safe levels of mycotoxins. J. Food Prot. 47:570.
Harrison, L.R., B.M. Colvin, J.T. Greene, L.E. Newman and R.J. Cole. 1990. Pulmonary edema and hydrothorax in swine produced by fumonisin B1, a toxic metabolite of Fusarium moniliforme. J Vet. Diagn. Invest. 2:217.
Harvey, R.B., L.F. Kubena, T.D. Phillips, W.E. Huff and D.E. Corrier. 1988. Approaches to the prevention of aflatoxicosis. Proc. Maryland Nutr. Conf. pp. 102-107.
Hayes, S.M. 1990. Counteracting aflatoxin in livestock feed. In: Agricultural Research, USDA, ARS, Washington, D.C. 38(2):18.
Hesseltine, C.W. 1986a. Global significance of mycotoxins. In: Mycotoxins and Phycotoxins. (P.S. Steyn and R. Vleggaar, eds). Elsevier Scientific Publishing Co., Amsterdam.
Hesseltine, C.W. 1986b. Resume´ and future needs in the diagnosis of mycotoxins. In: Diagnosis of Mycotoxicoses. (J.L. Richard and J.R. Thurston, eds.) Martinus Nijhoff Publishers, Dordrecht, The Netherlands.
Hsu, I.C., C.B. Smalley, F.M. Strong andW.E. Ribelin. 1972. Identification of T-2 toxin in moldy corn associated with a lethal toxicosis in dairy cattle. Appl. Microbiol. 24:684.
Ingalls, J.R. 1994. Influence of DON on feed consumption by dairy cows. In: Proc. Western Nutr. Conf. P 129. Winnipeg, MB, Canada. Joffe, A.Z. 1986. Fusarium Species: Their Biology and Toxicology. John Wiley and Sons, Inc., New York.
Kellela, K. and E. Ettala. 1984. The oestrogenic Fusarium toxin (zearalenone) in hay as a cause of early abortions in the cow. Nord. Vet. Med. 36:305.
Kellela, K. and L. Vasenius. 1982. The effects of rumen fluid on the content of zearalenone in animal fodder. Nord. Vet. Med. 34:336.
Khamis, Y., H.A. Hammad and N.A. Hemeida. 1986. Mycotoxicosis with oestrogenic effect in cattle. Zuchthyg. 21:233.
Kiessling, K.H., H. Patterson, K. Sandholm and M. Olsen. 1984. Metabolism of aflatoxin, ochratoxin, zearalenone, and three trichothecenes by intact rumen fluid, rumen protozoa and rumen bacteria. Appl. Environ. Microbiol. 47:1070.
Kosuri, N.R., M.D. Grave, S.G. Yates,W.H. Tallent, J.J. Ellis, I.A.Wolff and R.E. Nichols. 1970. Response of cattle to mycotoxins of Fusarium tricinctum isolated from corn and fescue. J. Am. Vet. Med. Assoc. 157:938.
Kriek, N.P.J., T.S. Kellerman and W.F.O. Marasas. 1981. A comparative study of the toxicity of Fusarium verticilloides (F. moniliforme) to horses, primates, pigs, sheep, and rats. Onderspoort J Vet. Res. 48:129.
Lacey, J. 1991. Natural occurrence of mycotoxins in growing and conserved forage crops. In: Mycotoxins and Animal Foods (J. E. Smith and R. E. Henderson, eds.), CRC Press, Boca Raton, Florida.
Lillehoj, E.B., and A. Ciegler. 1975. Mycotoxin synergism. In: Microbiology - 1975. (D. Schlessinger, ed.) American Society of Microbiology, Washington, DC. pp. 344-358.
Lindemann, M.D. and D.J. Blodgett. 1991. Various clays provide alternative for dealing with aflatoxin. Feedstuffs 63:15.
Mann, D.D., G.M. Buening, B. Hook and G.D. Osweiler. 1983. Effects of T-2 mycotoxin on bovine serum proteins. J. Am. Vet. Med. Assoc. 44:1757.
Marasas, W.F.O., T.S. Kellerman, W.C.A. Gelderblom, J.A.W. Coetzer, P.G. Thiel and J.J. van der Lugt. 1988. Leukoencephalomalacia in a horse induced by fumonisin B1 isolated from Fusarium moniliforme. Onderstepoort J. Vet Res 55:197.
Marsi, M.S., V.C. Garcia and J.R. Page. 1969. The aflatoxin M1 content of milk from cows fed known amounts of aflatoxin. Vet. Rec. 84:146.
Mills, J.T. and D. Abramson. 1986. Production of sterigmatocystin by isolates of Aspergillus versicolor from western Canadian stored barley and rapeseed/canola. Can. J. Plant Pathol. 8:151.
Mirocha, C.J., J.Harrison, A.A. Nichols and M. McClintock. 1968. Detection of fungal estrogen (F-2) in hay associated with infertility in dairy cattle. Appl. Microbiol. 16:797.
Mirocha, C.J., B. Schauerhamer and S.V. Pathre. 1974. Isolation, detection and quantitation of zearalenone in maize and barley. J. Assoc. Off. Anal. Chem. 57:1104.
Mirocha, C.J., S.V. Pathre, B. Schauerhamer and C.MChristiansen. 1976. Natural occurrence of Fusarium toxins in feedstuffs. Appl Environ. Microbiol. 32:553.
Moss, M.O. 1991. The environmental factors controllingmycotoxin formation. In: Mycotoxins and Animal Foods (J. E. Smith and R. S.
Henderson, eds.) CRC Press. Boca Raton, Florida. Nelson, M., N.R. Schneider, A.R. Doster, M.P. Carlson and T. Klopfenstein. 1984. Vomitoxin-contaminated wheat-pathology, toxicity in cattle. Nebraska Beef Cattle Report MP-47:3.
Noller, C.H., M. Stob and J. Tuite. 1979. Effects of feeding Gibberella zeae-infected corn on feed intake, bodyweight gain and milk production of dairy cows. J. Dairy Sci. 62:1003.
Osweiler, G.D., M.E. Kehrli, J.R. Stabel, J.R. Thurston, P.F. Ross and T.M. Wilson. 1993. Effects of fumonisin-contaminated corn screenings on growth and health of feeder calves. J. Anim. Sci. 71:459.
Patterson,D.S.P. and P.H. Anderson. 1982. Recent aflatoxin feeding experiments in cattle. Vet. Rec. 110:60.
Petrie, L., J. Robb and A.F. Stewart. 1977. The identification of T-2 toxin and its association with a hemorrhagic syndrome in cattle. Vet. Rec. 101:326.
Richard, J.L., G. Meerdink, C.M. Maragos,M. Tumbleson, G. Bordson, L.G. Rice and P.F. Ross. 1996. Absence of detectable fumonisins in the milk of cows fed Fusarium proliferatum (Matsushima) Nirenberg culture material. Mycopathologia 133:123.
Roine, K., E.L. Korpinen and K. Kallela. 1971. Mycotoxicosis as a probable cause of infertility in dairy cows. Nord. Vet. Med. 23:628.
Russel, L., D.F. Cox, G. Larsen, K. Bodwell and C.E Nelson. 1991. Incidence of molds and mycotoxins in commercial animal feed mills in seven Midwestern states, 1988-89. J. Anim. Sci. 69:5.
Schaeffer, J.L. and P.B. Hamilton. 1991. Interactions of mycotoxins with feed ingredients. Do safe levels exist? In: Mycotoxins and Animal Foods (J.E. Smith and R.S. Henderson, eds.) CRC Press. Boca Raton, Florida.
Scheideler, S.E. 1990. Aluminosilicates in poultry rations. Feed Management 41(1):22.
Schilfer,H.B. 1990. Mycotoxicosis of domestic animals and their diagnosis. Can. J. Physiol. Pharmacol. 68:987.
Scott, P.M. 1990. General referee reports: Mycotoxins. J. Assoc. Off. Anal. Chem. 73:98.
Scott, P.M., T. Delgado, D. B. Prelusky, H.L. Trenholm, J.D. Miller. 1994. Determination of fumonisin in milk. J. Environ. Sci. Health. B29:989.
Seglar, B. 1997. Case studies that implicate silage mycotoxins as the cause of dairy herd problems. In: Silage: Field to Feedbunk, NRAES-99, Northeast Regional Agricultural Engineering Service, Ithaca, NY. pp. 242.
Smith, T.K. 1980. Influence of dietary fiber, protein and zeolite on zearalenone toxicosis in rats and swine. J. Anim. Sci. 50:278.
Smith, T.K. 1984. Spent canola oil bleaching clays: potential for treatment of T-2 toxicosis in rats and short term inclusion in diets for immature swine. Can. J. Anim. Sci. 64:725.
Smith, T.K. and E.J. MacDonald. 1991. Effect of fusaric acid on brain regional neurochemistry and vomiting behavior in swine. J. Anim. Sci. 69:2044.
Smith, J., C. Wesselink, J. Parr, J.M. Sprosen, E.A. Fowke, N.R. Towers and D. Laboyrie. 1995. Effect of zearalenone on ewe pregnancy rates. In: Toxinology and Food Safety. Toxinology and Food Safety Research Group, Ruakura Research Centre, Hamilton, New Zealand.
Sprosen, J.M. And N.R. Towers. 1995. Urinary zearalenone metabolite concentrations in herds with fertility problems. In: Toxinology and Food Safety. Toxinology and Food Safety Research Group, Ruakura Research Centre, Hamilton, New Zealand.
Sreemannarayana, O., A.A. Frohlich, T.G. Vitti, R.R. Marquart and D. Abramson. 1988. Studies of the tolerance and disposition of ochratoxin A in young calves. J. Animal Sci. 66:1703.
Still, P., R.D.Wei, E.B. Smalley and F.M. Strong. 1972. Amycotoxin from Penicillium roqueforti isolated from toxic cattle feed. Fed. Proc. 31:733.
Sundlof, S.F. and C. Strickland. 1986. Zearalenone and zearalenol: Potential residue problems in livestock. Vet. Hum. Toxicol. 28:242.
Towers,N.R., J.M. Sprosen andW.Webber. 1995a. Zearalenone metabolites in cycling and non-cycling cows. In: Toxinology and Food Safety. Toxinology and Food SafetyResearch Group,RuakuraResearch Centre, Hamilton, New Zealand.
Towers, N.R., C.Wesselink, E.A. Fowke and J.M. Sprosen. 1995b. Plasma vs urinary ‘zearalenone’ concentrations as indicators of ‘zearalenone’ intake. In: Toxinology and Food Safety. Toxinology and Food Safety Research Group, Ruakura Research Centre, Hamilton, New Zealand.
Trenholm, H.L., B.K. Thompson, K.E. Hartin, R. Greenhalgh and A.J. McAllister. 1985. Ingestion of vomitoxin (deoxynivalenol)-contaminated wheat by nonlactating dairy cows. J. Dairy Sci. 68:1000.
Trenholm, H.L., D.B. Prelusky, J.C. Young and J.D. Miller. 1988. Reducing mycotoxins in animal feeds. Agriculture Canada Publication 1827E.
USDA. APHIS. 1995. Mycotoxin levels in the 1995 midwest preharvest corn crop. Veterinary Services Factsheet N195.1295. The National Veterinary Services Laboratory, Ames, Iowa.
Van Egmond, H.P. 1989. Mycotoxins in Dairy Products. Elsevier Science Pub. Co., Ltd. New York.
Vesonder, R.F., A. Ciegler, R.F. Rogers, K.A. Burbridge, R.J. Bothast and A.H. Jensen. 1978. Survey of 1977 crop year preharvest corn for vomitoxin. Appl. Environ. Microbiol. 36:885.
Vesonder, R.F. and B.W. Horn. 1985. Sterigmatocystin in dairy cattle feed contaminated with Aspergillus versicolor. Appl. Environ. Microbiol. 49:234.
Vough, L.R. and I. Glick. 1993. Round bale silage. In: Silage Production fromSeed to Animal,NARES-67, Northeast Regional Agricultural Engineering Service, Ithaca, NY. pp. 117.
Wannemacher, R.W., Jr., D.L. Brunner and H.A. Neufeld. 1991. Toxicity of trichothecenes and other relatedmycotoxins in laboratory animals. In: Mycotoxins and Animal Foods. (J. E. Smith and R. S. Henderson, eds.) CRC Press, Inc., Boca Raton, FL.
Weaver, G.A., H.J. Kurtz, C.J. Mirocha, F.Y. Bates, J.C. Behrens, T.S. Robison and S.P. Swanson. 1980. The failure of T-2 mycotoxin to produce hemorrhaging in dairy cattle. Can. Vet. J. 21:210.
Weaver, G.A., H.J. Kurtz, J.C. Behrens, T.S. Robison, B.E. Seguin, F.Y. Bates and C.J. Mirocha. 1986a. Effect of zearalenone on the fertility of virgin dairy heifers. Am. J. Vet. Res. 47:1395.
Weaver, G.A., H.J. Kurtz, J.C. Behrens, T.S. Robison, B.E. Seguin, F.Y. Bates and C.J. Mirocha. 1986b. Effect of zearalenone on dairy cows. Am. J. Vet. Res. 47:1826.
Whitlow, L.W., R.L. Nebel and W.M. Hagler, Jr. 1991. The association of deoxynivalenol in grain with milk production loss in dairy cows. In: G.C. Llewellyn,W.V. Dashek and C.E. O’Rear. 1994. Biodeterioration research 4. Plenum Press, New York. pp. 131.
Whitlow, L.W.,W.M. Hagler, Jr. and B.A. Hopkins. 1998. Mycotoxin occurrence in farmer-submitted samples of North Carolina feedstuffs: 1989- 1997. J. Dairy Sci. 81(Suppl. 1):1189.
Windels, H.F., A. DiCostanzo and R.D. Goodrich. 1995. Effect of deoxynivalenol from barley on performance and health of large frame crossbred steers. Minnesota Cattle Feeder Rep. B-417. St. Paul, MN. (As quoted by DiCostanzo et al., 1995a).
Wyatt, R.D. 1991. Measurement of mold growth and mycotoxins in feed: fallacies and innovations. In: Proceedings of the Georgia Nutrition Conference, University of Georgia, Athens.
Authors: LON W. WHITLOW1 and W. M. HAGLER, JR.2
1 Animal Science Department and
2 Poultry Science Department, North Carolina State University, Raleigh, North Carolina, USA







.jpg&w=3840&q=75)