Fumagillin, a Mycotoxin of Aspergillus fumigatus: Biosynthesis, Biological Activities, Detection, and Applications
Fumagillin is a mycotoxin produced, above all, by the saprophytic filamentous fungus Aspergillus fumigatus. This mold is an opportunistic pathogen that can cause invasive aspergillosis, a disease that has high mortality rates linked to it. Its ability to adapt to environmental stresses through the production of secondary metabolites, including several mycotoxins (gliotoxin, fumagillin, pseurotin A, etc.) also seem to play an important role in causing these infections. Since the discovery of the A. fumigatus fumagillin in 1949, many studies have focused on this toxin and in this review we gather all the information currently available. First of all, the structural characteristics of this mycotoxin and the different methods developed for its determination are given in detail. Then, the biosynthetic gene cluster and the metabolic pathway involved in its production and regulation are explained. The activity of fumagillin on its target, the methionine aminopeptidase type 2 (MetAP2) enzyme, and the effects of blocking this enzyme in the host are also described. Finally, the applications that this toxin and its derivatives have in different fields, such as the treatment of cancer and its microsporicidal activity in the treatment of honeybee hive infections with Nosema spp., are reviewed. Therefore, this work offers a complete review of all the information currently related to the fumagillin mycotoxin secreted by A. fumigatus, important because of its role in the fungal infection process but also because it has many other applications, notably in beekeeping, the treatment of infectious diseases, and in oncology.
Keywords: fumagillin; Aspergillus fumigatus; chemical detection; metabolic pathway and regulation; MetAP2 enzyme; cancer treatment; microsporicidal activity; honeybee hive infections
Key Contribution: Fumagillin is a mycotoxin produced during infections caused by A. fumigatus and in the adaptation processes of this species to multiple environmental stresses. In this review; we include detailed information on the fungal mechanisms employed in the production of this mycotoxin and those present in its regulation, how it acts on its target, and how it can be detected, all of which may be useful for diagnosis or the development of new treatments. Its usefulness in different fields is also described; because of its anti-angiogenic activity it is used to attack different types of tumors; at the same time its antibiotic activity is useful against several parasites.
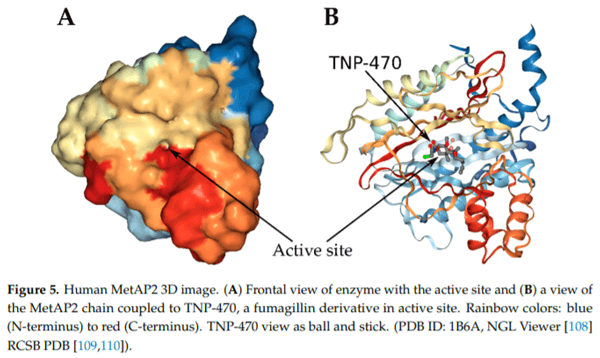
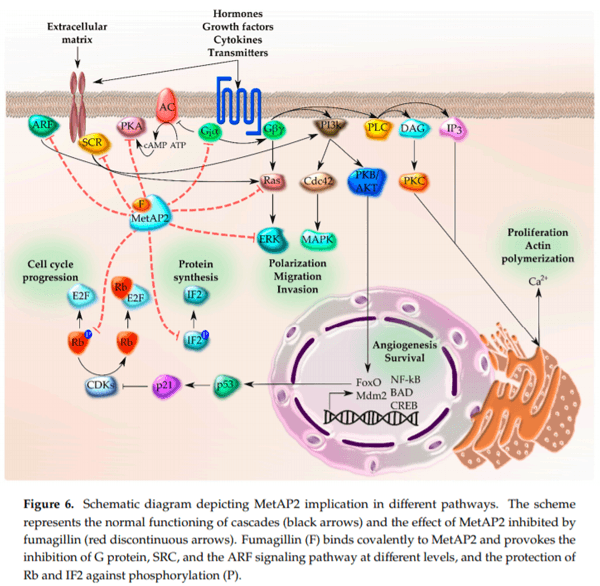
1. Latgé, J.P. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 1999, 12, 310–350. [CrossRef] [PubMed]
2. Tekaia, F.; Latgé, J.P. Aspergillus fumigatus: Saprophyte or pathogen? Curr. Opin. Microbiol. 2005, 8, 385–392. [CrossRef] [PubMed]
3. Dagenais, T.R.; Keller, N.P. Pathogenesis of Aspergillus fumigatus in Invasive Aspergillosis. Clin. Microbiol. Rev. 2009, 22, 447–465. [CrossRef] [PubMed]
4. Kwon-Chung, K.J.; Sugui, J.A. Aspergillus fumigatus—What makes the species a ubiquitous human fungal pathogen? PLoS Pathog. 2013, 9. [CrossRef]
5. Knox, B.P.; Blachowicz, A.; Palmer, J.M.; Romsdahl, J.; Huttenlocher, A.; Wang, C.C.; Keller, N.P.; Venkateswaran, K. Characterization of Aspergillus fumigatus isolates from air and surfaces of the international Space Station. mSphere 2016, 1, e00227-16. [CrossRef]
6. Paulussen, C.; Hallsworth, J.E.; Álvarez-Pérez, S.; Nierman, W.C.; Hamill, P.G.; Blain, D.; Rediers, H.; Lievens, B. Ecology of aspergillosis: Insights into the pathogenic potency of Aspergillus fumigatus and some other Aspergillus species. Microb. Biotechnol. 2017, 10, 296–322. [CrossRef]
7. Blachowicz, A.; Chiang, A.J.; Romsdahl, J.; Kalkum, M.; Wang, C.C.C.; Venkateswaran, K. Proteomic characterization of Aspergillus fumigatus isolated from air and surfaces of the International Space Station. Fungal Genet. Biol. 2019, 124, 39–46. [CrossRef]
8. Alshareef, F.; Robson, G.D. Prevalence, persistence, and phenotypic variation of Aspergillus fumigatus in the outdoor environment in Manchester, UK, over a 2-year period. Med. Mycol. 2014, 52, 367–375. [CrossRef]
9. Amarsaikhan, N.; O’Dea, E.M.; Tsoggerel, A.; Owegi, H.; Gillenwater, J.; Templeton, S.P. Isolate-dependent growth, virulence, and cell wall composition in the human pathogen Aspergillus fumigatus. PLoS ONE 2014, 9, 100430. [CrossRef] [PubMed]
10. Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4. [CrossRef] [PubMed]
11. Gauthier, G.M.; Keller, N.P. Crossover fungal pathogens: The biology and pathogenesis of fungi capable of crossing kingdoms to infect plants and humans. Fungal Genet. Biol. 2013, 61, 146–157. [CrossRef] [PubMed]
12. Raffa, N.; Keller, N.P. A call to arms: Mustering secondary metabolites for success and survival of an opportunistic pathogen. PLoS Pathog. 2019, 15, 1007606. [CrossRef] [PubMed]
13. Bennett, J.W.; Bentley, R. What’s in a name?—Microbial secondary metabolism. Adv. Appl. Microbiol. 1989, 34, 1–28. [CrossRef]
14. Arias, M.; Santiago, L.; Vidal-García, M.; Redrado, S.; Lanuza, P.; Comas, L.; Domingo, M.P.; Rezusta, A.; Gálvez, E.M. Preparations for invasion: Modulation of host lung immunity during pulmonary aspergillosis by gliotoxin and other fungal secondary metabolites. Front. Immunol. 2018, 9, 2549. [CrossRef] [PubMed]
15. Kamei, K.; Watanabe, A. Aspergillus mycotoxins and their effect on the host. Med. Mycol. 2005, 43, S95–S99. [CrossRef]
16. Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [CrossRef]
17. Keller, N.P.; Bok, J.W.; Chung, D.; Perrin, R.M.; Shwab, E.K. LaeA, a global regulator of Aspergillus toxins. Med. Mycol. 2006, 44, 83–85. [CrossRef]
18. Raffa, N.; Osherov, N.; Keller, N.P. Copper utilization, regulation, and acquisition by Aspergillus fumigatus. Int. J. Mol. Sci. 2019, 20, 1980. [CrossRef]
19. Romsdahl, J.; Wang, C.C.C. Recent advances in the genome mining of Aspergillus secondary metabolites (covering 2012–2018). Med. Chem. Commun. 2019, 10, 840–866. [CrossRef]
20. Shankar, J.; Tiwari, S.; Shishodia, S.K.; Gangwar, M.; Hoda, S.; Thakur, R.; Vijayaraghavan, P. Molecular insights into development and virulence determinants of Aspergilli: A proteomic perspective. Front. Cell. Infect. Microbiol. 2018, 8, 180. [CrossRef]
21. Frisvad, J.C.; Rank, C.; Nielsen, K.F.; Larsen, T.O. Metabolomics of Aspergillus fumigatus. Med. Mycol. 2009, 47, 53–71. [CrossRef]
22. Keller, N.P.; Turner, G.; Bennett, J.W. Fungal secondary metabolism—from biochemistry to genomics. Nat. Rev. Microbiol. 2005, 3, 937–947. [CrossRef]
23. Bignell, E.; Cairns, T.C.; Throckmorton, K.; Nierman, W.C.; Keller, N.P. Secondary metabolite arsenal of an opportunistic pathogenic fungus. Philos Trans R. Soc. B 2016, 371, 20160023. [CrossRef]
24. Lind, A.L.; Wisecaver, J.H.; Lameiras, C.; Wiemann, P.; Palmer, J.M.; Keller, N.P.; Rodrigues, F.; Goldman, G.H.; Rokas, A. Drivers of genetic diversity in secondary metabolic gene clusters within a fungal species. PLoS Biol. 2017, 15. [CrossRef]
25. Lind, A.L.; Lim, F.Y.; Soukup, A.A.; Keller, N.P.; Rokas, A. An LaeA- and BrlA-dependent cellular network governs tissue-specific secondary metabolism in the human pathogen Aspergillus fumigatus. mSphere 2018, 3. [CrossRef]
26. Hanson, F.R.; Eble, T.E. An antiphage agent isolated from Aspergillus sp. J. Bacteriol. 1949, 58, 527–529.
27. Perrin, R.M.; Fedorova, N.D.; Bok, J.W.; Cramer, R.A.; Wortman, J.R.; Kim, H.S.; Nierman, W.C.; Keller, N.P. Transcriptional regulation of chemical diversity in Aspergillus fumigatus by LaeA. PLoS Pathog. 2007, 3, 50. [CrossRef]
28. Wiemann, P.; Guo, C.; Palmer, J.M.; Sekonyela, R.; Wang, C.C.C.; Keller, N.P. Prototype of an intertwined secondary-metabolite supercluster. Proc. Natl. Acad. Sci. USA 2013, 110, 17065–17070. [CrossRef]
29. Sin, N.; Meng, L.; Wang, M.Q.; Wen, J.J.; Bornmann, W.G.; Crews, C.M. The anti-angiogenic agent fumagillin covalently binds and inhibits the methionine aminopeptidase, MetAP-2. Proc. Natl. Acad. Sci. USA 1997, 94, 6099–6103. [CrossRef]
30. Mauriz, J.L.; Martín-Renedo, J.; García-Palomo, A.; Tuñón, M.J.; González-Gallego, J. Methionine aminopeptidases as potential targets for treatment of gastrointestinal cancers and other tumours. Curr. Drug Targets 2010, 11, 1439–1457. [CrossRef]
31. Vetro, J.A.; Dummitt, B.; Chang, Y.H. Methionine-aminopeptidase: Emerging role in angiogenesis. In Aminopeptidases in Biology and Disease; Hooper, N.M., Lendeckel, U., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2004; pp. 17–44.
32. Arico-Muendel, C.; Centrella, P.A.; Contonio, B.D.; Morgan, B.A.; O’Donovan, G.; Paradise, C.L.; Skinner, S.R.; Sluboski, B.; Svendsen, J.L.; White, K.F.; et al. Antiparasitic activities of novel, orally available fumagillin analogs. Bioorganic Med. Chem. Lett. 2009, 19, 5128–5131. [CrossRef]
33. Novohradska, S.; Ferling, I.; Hillmann, F. Exploring Virulence Determinants of Filamentous Fungal Pathogens through Interactions with Soil Amoebae. Front. Cell. Infect. Microbiol. 2017, 7, 497. [CrossRef]
34. Casadevall, A.; Fu, M.S.; Guimaraes, A.J.; Albuquerque, P. The ‘Amoeboid Predator-Fungal Animal Virulence’ Hypothesis. J. Fungi 2019, 5, 10. [CrossRef]
35. Molina, J.M.; Tourneur, M.; Sarfati, C.; Chevret, S.; de Gouvello, A.; Gobert, J.G.; Balkan, S.; Derouin, F. Fumagillin treatment of intestinal microsporidiosis. N. Engl. J. Med. 2002, 346, 1963–1969. [CrossRef]
36. van den Heever, J.P.; Thompson, T.S.; Curtis, J.M.; Ibrahim, A.; Pernal, S.F. Fumagillin: An overview of recent scientific advances and their significance for apiculture. J. Agric. Food Chem. 2014, 62, 2728–2737. [CrossRef]
37. van den Heever, J.P.; Thompson, T.S.; Otto, S.J.G.; Curtis, J.M.; Ibrahim, A.; Pernal, S.F. Evaluation of Fumagilin-B and other potential alternative chemotherapies against Nosema ceranae-infected honeybees (Apis mellifera) in cage trial assays. Apidologie 2016, 47, 617–630. [CrossRef]
38. Fallon, J.P.; Reeves, E.P.; Kavanagh, K. Inhibition of neutrophil function following exposure to the Aspergillus fumigatus toxin fumagillin. J. Med. Microbiol. 2010, 59, 625–633. [CrossRef]
39. Zbidah, M.; Lupescu, A.; Jilani, K.; Lang, F. Stimulation of suicidal erythrocyte death by fumagillin. Basic Clin. Pharmacol. Toxicol. 2013, 112, 346–351. [CrossRef]
40. Guruceaga, X.; Ezpeleta, G.; Mayayo, E.; Sueiro-Olivares, M.; Abad-Diaz-De-Cerio, A.; Aguirre Urizar, J.M.; Liu, H.G.; Wiemann, P.; Bok, J.W.; Filler, S.G.; et al. A possible role for fumagillin in cellular damage during host infection by Aspergillus fumigatus. Virulence 2018, 9, 1548–1561. [CrossRef]
41. MarvinSketch, (version 17.22.0, calculation module developed by ChemAxon, 2017).
42. DrugBank. Available online: https://www.drugbank.ca/about (accessed on 6 November 2019).
43. Log D Predictor. Available online: https://disco.chemaxon.com/apps/demos/logd/ (accessed on 15 October 2019).
44. Garrett, E.R.; Eble, T.E. Studies on the stability of fumagillin. I. Photolytic degradation in alcohol solution. J. Am. Pharm. Assoc. Sci. Ed. 1954, 43, 385–390. [CrossRef]
45. Eble, T.E.; Garrett, E.R. Studies on the stability of fumagillin. II. Photolytic degradation of crystalline fumagillin. J. Am. Pharm. Assoc. Sci. Ed. 1954, 43, 536–538. [CrossRef]
46. Garrett, E.R. Studies on the stability of fumagillin. III. Thermal degradation in the presence and absence of air. J. Am. Pharm. Assoc. Sci. Ed. 1954, 43, 539–543. [CrossRef]
47. Brackett, J.M.; Arguello, M.D.; Schaar, J.C. Determination of fumagillin by high-performance liquid-chromatography. J. Agric. Food Chem. 1988, 36, 762–764. [CrossRef]
48. Kochansky, J.; Nasr, M. Laboratory studies on the photostability of fumagillin, the active ingredient of Fumidil B. Apidologie 2004, 35, 301–310. [CrossRef]
49. Dmitrovic, J.; Durden, D.A. Analysis of fumagillin in honey by LC-MS/MS. J. AOAC Int. 2013, 96, 687–695. [CrossRef]
50. Assil, H.I.; Sporns, P. ELISA and HPLC methods for analysis of fumagillin and its decomposition products in honey. J. Agric. Food Chem. 1991, 39, 2206–2213. [CrossRef]
51. Higes, M.; Nozal, M.J.; Alvaro, A.; Barrios, L.; Meana, A.; Martin-Hernandez, R.; Bernal, J.L.; Bernal, J. The stability and effectiveness of fumagillin in controlling Nosema ceranae (Microsporidia) infection in honey bees (Apis mellifera) under laboratory and field conditions. Apidologie 2011, 42, 364–377. [CrossRef]
52. Van den Heever, J.P.; Thompson, T.S.; Curtis, J.M.; Pernal, S.F. Stability of dicyclohexylamine and fumagillin in honey. Food Chem. 2015, 179, 152–158. [CrossRef]
53. Nozal, M.J.; Bernal, J.L.; Martin, M.T.; Bernal, J.; Alvaro, A.; Martin, R.; Higes, M. Trace analysis of fumagillin in honey by liquid chromatography-diode array-electrospray ionization mass spectrometry. J. Chromatogr. A 2008, 1190, 224–231. [CrossRef]
54. Van den Heever, J.P.; Thompson, T.S.; Curtis, J.M.; Pernal, S.F. Determination of dicyclohexylamine and fumagillin in Honey by LC-MS/MS. Food Anal. Meth. 2015, 8, 767–777. [CrossRef]
55. Lopez, M.I.; Pettis, J.S.; Smith, I.B.; Chu, P.S. Multiclass determination and confirmation of antibiotic residues in honey using LC-MS/MS. J. Agric. Food Chem. 2008, 56, 1553–1559. [CrossRef] [PubMed]
56. Iveši´c, M.; Krivohlavek, A.; Žuntar, I.; Toli´c, S.; Šiki´c, S.; Musi´c, V.; Pavli´c, I.; Bursik, A.; Gali´c, N. Monitoring of selected pharmaceuticals in surface waters of Croatia. Environ. Sci. Pollut. Res. 2017, 24, 23389–23400. [CrossRef] [PubMed]
57. Jia, W.; Chua, X.; Ling, Y.; Huang, J.; Chang, J. Multi-mycotoxin analysis in dairy products by liquid chromatography coupled to quadrupole orbitrap mass spectrometry. J. Chromatogr. A 2014, 1345, 107–114. [CrossRef] [PubMed]
58. Kanda, M.; Sasamoto, T.; Takeba, K.; Hayashi, H.; Kusano, T.; Matsushima, Y.; Nakajima, T.; Kanai, S.; Takano, I. Rapid Determination of Fumagillin Residues in Honey by Liquid Chromatography-Tandem Mass Spectrometry Using the QuEChERS Method. J. AOAC Int. 2011, 94, 878–885.
59. Guyonnet, J.; Richard, M.; Hellings, P. Determination of fumagillin in muscle-tissue of rainbow-trout using automated ion-pairing liquid-chromatography. J. Chromatogr. B-Biomed. Appl. 1995, 666, 354–359. [CrossRef]
60. Fekete, J.; Romvari, Z.; Szepesi, I.; Morovjan, G. Liquid-chromatographic determination of the antibiotic fumagillin in fish meat samples. J. Chromatogr. A 1995, 712, 378–381. [CrossRef]
61. Fekete, J.; Romvari, Z.; Gebefugi, I.; Kettrup, A. Comparative study on determination of fumagillin in fish by normal and reversed phase chromatography. Chromatographia 1998, 48, 48–52. [CrossRef]
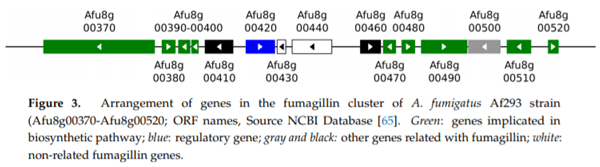
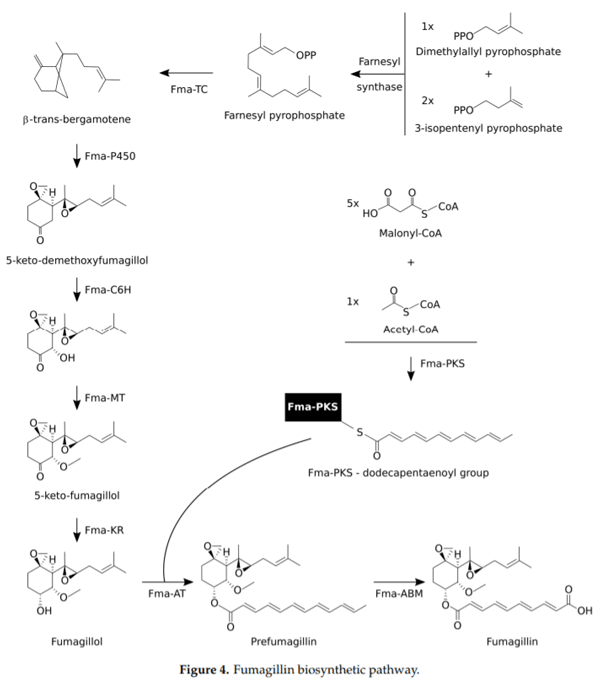
62. Lin, H.C.; Chooi, Y.H.; Dhingra, S.; Xu, W.; Calvo, A.M.; Tang, Y.J. The fumagillin biosynthetic gene cluster in Aspergillus fumigatus encodes a cryptic terpene cyclase involved in the formation of β-trans-bergamotene. J. Am. Chem. Soc. 2013, 135, 4616–4619. [CrossRef]
63. Lin, H.C.; Tsunematsu, Y.; Dhingra, S.; Xu, W.; Fukutomi, M.; Chooi, Y.H.; Cane, D.E.; Calvo, A.M.; Watanabe, K.; Tang, Y. Generation of complexity in fungal terpene biosynthesis, discovery of a multifunctional cytochrome P450 in the fumagillin pathway. J. Am. Chem. Soc. 2014, 136, 4426–4436. [CrossRef]
64. Dhingra, S.; Lind, A.L.; Lin, H.C.; Tang, Y.; Rokas, A.; Calvo, A.M. The fumagillin gene cluster, an example of hundreds of genes under veA control in Aspergillus fumigatus. PLoS ONE 2013, 8, 77147. [CrossRef]
65. NCBI (National Center for Biotechnology Information). Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 6 November 2019).
66. AspGD (Aspergillus Genome Database). Available online: http://www.aspgd.org/ (accessed on 6 November 2019).
67. UniProtKB (UniProt Knowledgebase). Available online: https://www.uniprot.org/ (accessed on 6 November 2019).
68. Gravelat, F.N.; Doedt, T.; Chiang, L.Y.; Liu, H.; Filler, S.G.; Patterson, T.F.; Sheppard, D.C. In Vivo analysis of Aspergillus fumigatus developmental gene expression determined by real-time reverse transcription-PCR. Infect. Immun. 2008, 76, 3632–3639. [CrossRef]
69. Vödisch, M.; Scherlach, K.; Winkler, R.; Hertweck, C.; Braun, H.P.; Roth, M.; Haas, H.; Werner, E.R.; Brakhage, A.A.; Kniemeyer, O. Analysis of the Aspergillus fumigatus proteome reveals metabolic changes and the activation of the pseurotin A biosynthesis gene cluster in response to hypoxia. J. Proteome Res. 2011, 10, 2508–2524. [CrossRef]
70. Mascotti, M.L.; Ayub, M.J.; Dudek, H.; Sanz, M.K.; Fraaije, M.W. Cloning, overexpression and biocatalytic exploration of a novel Baeyer-Villiger monooxygenase from Aspergillus fumigatus Af293. AMB Express 2013, 3, 33. [CrossRef]
71. Tsunematsu, Y.; Fukutomi, M.; Saruwatari, T.; Noguchi, H.; Hotta, K.; Tang, Y.; Watanabe, K. Elucidation of pseurotin biosynthetic pathway points to trans-acting C-methyltransferase: Generation of chemical diversity. Angew. Chem. Int. Ed. 2014, 53, 8475–8479. [CrossRef]
72. Bruns, S.; Seidler, M.; Albrecht, D.; Salvenmoser, S.; Remme, N.; Hertweck, C.; Brakhage, A.A.; Kniemeyer, O.; Müller, F.M. Functional genomic profiling of Aspergillus fumigatus biofilm reveals enhanced production of the mycotoxin gliotoxin. Proteomics 2010, 10, 3097–3107. [CrossRef]
73. Lind, A.L.; Smith, T.D.; Saterlee, T.; Calvo, A.M.; Rokas, A. Regulation of secondary metabolism by the Velvet complex is temperature-responsive in Aspergillus. G3 Bethesda 2016, 6, 4023–4033. [CrossRef]
74. Calvo, A.M.; Wilson, R.A.; Bok, J.W.; Keller, N.P. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 2002, 66, 447–459. [CrossRef]
75. Brakhage, A.A. Regulation of fungal secondary metabolism. Nat. Rev. Microbiol. 2013, 11, 21–32. [CrossRef]
76. Bok, J.W.; Keller, N.P. LaeA, a regulator of secondary metabolism in Aspergillus spp. Eukaryot Cell 2004, 3, 527–535. [CrossRef]
77. Bok, J.W.; Balajee, S.A.; Marr, K.A.; Andes, D.; Nielsen, K.F.; Frisvad, J.C.; Keller, N.P. LaeA, a regulator of morphogenetic fungal virulence factors. Eukaryot Cell 2005, 4, 1574–1582. [CrossRef]
78. Bayram, O.; Krappmann, S.; Ni, M.; Bok, J.W.; Helmstaedt, K.; Valerius, O.; Braus-Stromeyer, S.; Kwon, N.J.; Keller, N.P.; Yu, J.H.; et al. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science 2008, 320, 1504–1506. [CrossRef]
79. Amaike, S.; Keller, N.P. Distinct roles for VeA and LaeA in development and pathogenesis of Aspergillus flavus. Eukaryot Cell 2009, 8, 1051–1060. [CrossRef]
80. Gehrke, A.; Heinekamp, T.; Jacobsen, I.D.; Brakhage, A.A. Heptahelical receptors GprC and GprD of Aspergillus fumigatus are essential regulators of colony growth, hyphal morphogenesis, and virulence. Appl. Environ. Microbiol. 2010, 76, 3989–3998. [CrossRef]
81. Kim, S.S.; Kim, Y.H.; Shin, K.S. The developmental regulators, FlbB and FlbE, are involved in the virulence of Aspergillus fumigatus. J. Microbiol. Biotechnol. 2013, 23, 766–770. [CrossRef]
82. Johns, A.; Scharf, D.H.; Gsaller, F.; Schmidt, H.; Heinekamp, T.; Straßburger, M.; Oliver, J.D.; Birch, M.; Beckmann, N.; Dobb, K.S.; et al. A nonredundant phosphopantetheinyl transferase, PptA, is a novel antifungal target that directs secondary metabolite, siderophore, and lysine biosynthesis in Aspergillus fumigatus and is critical for pathogenicity. MBio 2017, 8. [CrossRef]
83. Manfiolli, A.O.; de Castro, P.A.; Dos Reis, T.F.; Dolan, S.; Doyle, S.; Jones, G.; Riaño Pachón, D.M.; Ula¸s, M.; Noble, L.M.; Mattern, D.J.; et al. Aspergillus fumigatus protein phosphatase PpzA is involved in iron assimilation, secondary metabolite production, and virulence. Cell. Microbiol. 2017, 19. [CrossRef]
84. Wu, M.Y.; Mead, M.E.; Lee, M.K.; Ostrem Loss, E.M.; Kim, S.C.; Rokas, A.; Yu, J.H. Systematic dissection of the evolutionarily conserved WetA developmental regulator across a genus of filamentous fungi. mBio 2018, 9. [CrossRef]
85. Lind, A.L.; Wisecaver, J.H.; Smith, T.D.; Feng, X.; Calvo, A.M.; Rokas, A. Examining the evolution of the regulatory circuit controlling secondary metabolism and development in the fungal genus Aspergillus. PLoS Genet. 2015, 11. [CrossRef]
86. Yu, Y.; Blachowicz, A.; Will, C.; Szewczyk, E.; Glenn, S.; Gensberger-Reigl, S.; Nowrousian, M.; Wang, C.C.C.; Krappmann, S. Mating-type factor-specific regulation of the fumagillin/pseurotin secondary metabolite supercluster in Aspergillus fumigatus. Mol. Microbiol. 2018, 110, 1045–1065. [CrossRef]
87. Conrad, T.; Kniemeyer, O.; Henkel, S.G.; Krüger, T.; Mattern, D.J.; Valiante, V.; Guthke, R.; Jacobsen, I.D.; Brakhage, A.A.; Vlaic, S.; et al. Module-detection approaches for the integration of multilevel omics data highlight the comprehensive response of Aspergillus fumigatus to caspofungin. BMC Syst. Biol. 2018, 1, 88. [CrossRef]
88. Eshwika, A.; Kelly, J.; Fallon, J.P.; Kavanagh, K. Exposure of Aspergillus fumigatus to caspofungin results in the release, and de novo biosynthesis, of gliotoxin. Med. Mycol. 2013, 51, 121–127. [CrossRef]
89. Netzker, T.; Fischer, J.; Weber, J.; Mattern, D.J.; König, C.C.; Valiante, V.; Schroeckh, V.; Brakhage, A.A. Microbial communication leading to the activation of silent fungal secondary metabolite gene clusters. Front. Microbiol. 2015, 6, 299. [CrossRef]
90. Arfin, S.M.; Kendall, R.L.; Hall, L.; Weaver, L.H.; Stewart, A.E.; Matthews, B.W.; Bradshaw, R.A. Eukaryotic methionyl aminopeptidases: Two classes of cobalt-dependent enzymes. Proc. Natl. Acad. Sci. USA 1995, 92, 7714–7718. [CrossRef]
91. Li, X.; Chang, Y.H. Evidence that the human homologue of a rat initiation factor-2 associated protein (p67) is a methionine aminopeptidase. Biochem. Biophys. Res. Commun. 1996, 227, 152–159. [CrossRef]
92. Meinnel, T.; Mechulam, Y.; Blanquet, S. Methionine as translation start signal: A review of the enzymes of the pathway in Escherichia coli. Biochimie 1993, 75, 1061–1075. [CrossRef]
93. Boissel, J.P.; Kasper, T.J.; Shah, S.C.; Malone, J.I.; Bunn, H.F. Amino-terminal processing of proteins: Hemoglobin South Florida, a variant with retention of initiator methionine and N(α)-acetylation. Proc. Natl. Acad. Sci. USA 1985, 82, 8448–8452. [CrossRef]
94. Chang, Y.H.; Teichert, U.; Smith, J.A. Molecular cloning, sequencing, deletion, and overexpression of a methionine aminopeptidase gene from Saccharomyces cerevisiae. J. Biol. Chem. 1992, 267, 8007–8011.
95. Chen, S.; Vetro, J.A.; Chang, Y.H. The specificity in vivo of two distinct methionine aminopeptidases in Saccharomyces cerevisiae. Arch. Biochem. Biophys. 2002, 398, 87–93. [CrossRef]
96. Walker, K.W.; Bradshaw, R.A. Yeast Methionine Aminopeptidase, I. Alteration of substrate specificity by site-directed mutagenesis. J. Biol. Chem. 1999, 274, 13403–13409. [CrossRef]
97. Catalano, A.; Romano, M.; Robuffo, I.; Strizzi, L.; Procopio, A. Methionine aminopeptidase-2 regulates human mesothelioma cell survival: Role of Bcl-2 expression and telomerase activity. Am. J. Pathol. 2001, 159, 721–731. [CrossRef]
98. Lowther, W.T.; Matthews, B.W. Structure and function of the methionine aminopeptidases. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 2000, 1477, 157–167. [CrossRef]
99. Endo, H.; Takenaga, K.; Kanno, T.; Satoh, H.; Mori, S. Methionine aminopeptidase 2 is a new target for the metastasis-associated protein, S100A4. J. Biol. Chem. 2002, 277, 26396–26402. [CrossRef]
100. Bradshaw, R.A.; Brickey, W.W.; Walker, K.W. N-terminal processing: The methionine aminopeptidase and N(α)-acetyl transferase families. Trends Biochem. Sci. 1998, 23, 263–267. [CrossRef]
101. Ray, M.K.; Datta, B.; Chakraborty, A.; Chattopadhyay, A.; Meza-Keuthen, S.; Gupta, N.K. The eukaryotic initiation factor 2-associated 67-kDa polypeptide (p67) plays a critical role in regulation of protein synthesis initiation in animal cells. Proc. Natl. Acad. Sci. USA 1992, 89, 539–543. [CrossRef]
102. Datta, B.; Ray, M.K.; Chakrabarti, D.; Wylie, D.E.; Gupta, N.K. Glycosylation of eukaryotic peptide chain initiation factor 2 (eIF-2)-associated 67-kDa polypeptide (p67) and its possible role in the inhibition of eIF-2 kinase-catalyzed phosphorylation of the eIF-2 α-subunit. J. Biol. Chem. 1989, 264, 20620–20624.
103. Atanassova, A.; Sugita, M.; Sugiura, M.; Pajpanova, T.; Ivanov, I. Molecular cloning, expression and characterization of three distinctive genes encoding methionine aminopeptidases in cyanobacterium Synechocystis sp. strain PCC6803. Arch. Microbio. 2003, 180, 185–193. [CrossRef]
104. Giglione, C.; Serero, A.; Pierre, M.; Boisson, B.; Meinnel, T. Identification of eukaryotic peptide deformylases reveals universality of N-terminal protein processing mechanisms. EMBO J. 2000, 19, 5916–5929. [CrossRef]
105. Leszczyniecka, M.; Bhatia, U.; Cueto, M.; Nirmala, N.R.; Towbin, H.; Vattay, A.; Wang, B.; Zabludoff, S.; Phillips, P.E. MAP1D, a novel methionine aminopeptidase family member is overexpressed in colon cancer. Oncogene 2006, 25, 3471–3478. [CrossRef]
106. Ingber, D.; Fujita, T.; Kishimoto, S.; Kanamaru, T.; Brem, H.; Folkman, J. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature 1990, 348, 555–557. [CrossRef]
107. Liu, S.; Widom, J.; Kemp, C.W.; Crews, C.M.; Clardy, J. Structure of Human Methionine Aminopeptidase-2 complexed with Fumagillin. Science 1998, 282, 1324–1328. [CrossRef]
108. Rose, A.S.; Bradley, A.R.; Valasatava, Y.; Duarte, J.M.; Prli´c, A.; Rose, P.W. NGL viewer: Web-based molecular graphics for large complexes. Bioinformatics 2018, 34, 3755–3758. [CrossRef]
109. RCSB PDB (RCSB Protein Data Bank). Available online: http://www.rcsb.org/3d-view/1B6A (accessed on 21 November 2019).
110. Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [CrossRef]
111. Datta, R.; Choudhury, P.; Bhattacharya, M.; Soto Leon, F.; Zhou, Y.; Datta, B. Protection of translation initiation factor eIF2 phosphorylation correlates with eIF2-associated glycoprotein p67 levels and requires the lysine-rich domain I of p67. Biochimie 2001, 83, 919–931. [CrossRef]
112. Buscà, R.; Pouysségur, J.; Lenormand, P. ERK1 and ERK2 map kinases: Specific roles or functional redundancy? Front. Cell. Dev. Biol. 2016, 4, 1–23. [CrossRef]
113. Datta, B.; Majumdar, A.; Datta, R.; Balusu, R. Treatment of cells with the angiogenic inhibitor fumagillin results in increased stability of eukaryotic initiation factor 2-associated glycoprotein, p67, and reduced phosphorylation of extracellular signal-regulated kinases. Biochemistry 2004, 43, 14821–14831. [CrossRef]
114. Datta, R.; Choudhury, P.; Ghosh, A.; Datta, B. A glycosylation site, 60SGTS63, of p67 is required for its ability to regulate the phosphorylation and activity of eukaryotic initiation factor 2α. Biochemistry 2003, 42, 5453–5460. [CrossRef]
115. Bernier, S.G.; Taghizadeh, N.; Thompson, C.D.; Westlin, W.F.; Hannig, G. Methionine aminopeptidases type I and type II are essential to control cell proliferation. J. Cell. Biochem. 2005, 95, 1191–1203. [CrossRef]
116. Sawanyawisuth, K.; Wongkham, C.; Pairojkul, C.; Saeseow, O.T.; Riggins, G.J.; Araki, N.; Wongkham, S. Methionine aminopeptidase 2 over-expressed in cholangiocarcinoma: Potential for drug target. Acta Oncol. 2007, 46, 378–385. [CrossRef]
117. Kanno, T.; Endo, H.; Takeuchi, K.; Moristhita, Y.; Fukayama, M.; Mori, S. High expression of methionine aminopeptidase type 2 in germinal center B cells and their neoplastic counterparts. Lab. Investig. 2002, 82, 893–901. [CrossRef]
118. Chang, S.Y.P.; McGary, E.C.; Chang, S. Methionine aminopeptidase gene of Escherichia coli is essential for cell growth. J. Bacteriol. 1989, 171, 4071–4072. [CrossRef]
119. Miller, C.G.; Strauch, K.L.; Kukral, A.M.; Miller, J.L.; Wingfield, P.T.; Mazzei, G.J.; Werlen, R.C.; Graber, P.; Movva, N.R. N-terminal methionine-specific peptidase in Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 1987, 84, 2718–2722. [CrossRef]
120. Li, X.; Chang, Y.H. Amino-terminal protein processing in Saccharomyces cerevisiae is an essential function that requires two distinct methionine aminopeptidases. Proc. Natl. Acad. Sci. USA 1995, 92, 12357–12361. [CrossRef]
121. Keller, N.P. Translating biosynthetic gene clusters into fungal armor and weaponry. Nat. Chem. Biol. 2015, 11, 671–677. [CrossRef]
122. Buss, J.E.; Mumby, S.M.; Casey, P.J.; Gilman, A.G.; Sefton, B.M. Myristoylated alpha subunits of guanine nucleotide-binding regulatory proteins. Proc. Natl. Acad. Sci. USA 1987, 84, 7493–7497. [CrossRef]
123. Yang, Z.; Wensel, T.G. N-Myristoylation of the rod outer segment G protein, transducin, in cultured retinas. J. Biol. Chem. 1992, 267, 23197–23201.
124. Neves, S.R.; Ram, P.T.; Iyengar, R. G protein pathways. Science 2002, 296, 1636–1639. [CrossRef]
125. Van Keulen, S.C.; Rothlisberger, U. Effect of N-terminal myristoylation on the active conformation of Gαi1 -GTP. Biochemistry 2017, 56, 271–280. [CrossRef]
126. Gresset, A.; Sondek, J.; Harden, T.K. The phospholipase C isozymes and their regulation. Subcellular Biochemistry. In Phosphoinositides I: Enzymes of Synthesis and Degradation; Balla, T., Wymann, M., York, J., Eds.; Springer: Dordrecht, The Netherlands, 2012; Volume 58, pp. 61–94.
127. Xu, J.; Wang, F.; Van Keymeulen, A.; Herzmark, P.; Straight, A.; Kelly, K.; Takuwa, Y.; Sugimoto, N.; Mitchison, T.; Bourne, H.R. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell 2003, 114, 201–214. [CrossRef]
128. Manning, B.D.; Toker, A. AKT/PKB signaling: Navigating the network. Cell 2018, 169, 381–405. [CrossRef]
129. Roskoski, R., Jr. Src protein–tyrosine kinase structure and regulation. Biochem. Biophys. Res. Commun. 2004, 324, 1155–1164. [CrossRef]
130. Tillo, S.E.; Xiong, W.H.; Thakahashi, M.; Miao, S.; Andrade, A.L.; Fortin, D.A.; Yang, G.; Qin, M.; Smoody, B.F.; Stork, P.J.S.; et al. Liberated PKA catalytic subunits associate with the membrane via myristoylation to preferentially phosphorylate membrane substrates. Cell Rep. 2017, 19, 617–629. [CrossRef]
131. Behnia, R.; Munro, S. Organelle identity and the signposts for membrane traffic. Nature 2005, 438, 597–604. [CrossRef]
132. Aitken, A.; Cohen, P.; Santikarn, S.; Williams, D.H.; Calder, A.G.; Smith, A.; Kleet, C.B. Identification of the NHZ-terminal blocking group of calcineurin B as myristic acid. FEBS Lett. 1982, 150, 314–318. [CrossRef]
133. D’Souza-Schorey, C.; Chavrier, P. ARF proteins: Roles in membrane traffic and beyond. Nat. Rev. Mol. Cell Biol. 2006, 7, 347–358. [CrossRef]
134. Mahajan-Thakur, S.; Bien-Möller, S.; Marx, S.; Schroeder, H.; Rauch, B.H. Sphingosine 1-phosphate (S1P) signaling in glioblastoma multiforme—A systematic review. Int. J. Mol. Sci. 2017, 18, 2448. [CrossRef]
135. Erpel, T.; Courtneidge, S.A. Src family protein tyrosine kinases and cellular signal transduction pathways. Curr. Opin. Cell Biol. 1995, 7, 176–182. [CrossRef]
136. Warder, S.E.; Tucker, L.A.; Mcloughlin, S.M.; Strelitzer, T.J.; Meuth, J.L.; Zhang, Q.; Sheppard, G.S.; Richardson, P.L.; Lesniewski, R.; Davidsen, S.K.; et al. Discovery, identification, and characterization of candidate pharmacodynamic markers of methionine aminopeptidase-2 inhibition. J. Proteome Res. 2008, 7, 4807–4820. [CrossRef]
137. Frottin, F.; Bienvenut, W.V.; Bignon, J.; Jacquet, E.; Vaca Jacome, A.S.; Van Dorsselaer, A.; Cianferani, S.; Carapito, C.; Meinnel, T.; Giglione, C.; et al. MetAP1 and MetAP2 drive cell selectivity for a potent anti-cancer agent in synergy, by controlling glutathione redox state. Oncotarget 2016, 7, 63306–63323. [CrossRef]
138. Okrój, M.; Kamysz, W.; Slominska, E.M.; Mysliwski, A.; Bigda, J. A novel mechanism of action of the fumagillin analog, TNP-470, in the B16F10 murine melanoma cell line. Anticancer Drugs 2005, 16, 817–823. [CrossRef]
139. Liu, Y.; Kahn, R.A.; Prestegard, J.H. Structure and Membrane Interaction of Myristoylated ARF1. Structure 2009, 17, 79–87. [CrossRef]
140. Abad, A.; Fernández-Molina, J.V.; Bikandi, J.; Ramírez, A.; Margareto, J.; Sendino, J.; Hernando, F.L.; Pontón, J.; Garaizar, J.; Rementeria, A. What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Rev. Iberoam. Micol. 2010, 27, 155–182. [CrossRef] [PubMed]
141. Sigg, H.P.; Weber, H.P. Isolierung und strukturaufklarung von ovalicin. Helv. Chim. Acta 1968, 51, 1395–1408. [CrossRef]
142. Griffith, E.C.; Su, Z.; Turk, B.E.; Chen, S.; Chang, Y.H.; Wu, Z.; Biemann, K.; Liu, J.O. Methionine aminopeptidase (type 2) is the common target for angiogenesis inhibitors AGM-1470 and ovalicin. Chem. Biol. 1997, 4, 461–471. [CrossRef]
143. Mangmool, S.; Kurose, H. Gi/o protein-dependent and -independent actions of pertussis toxin (PTX). Toxins 2011, 3, 884–899. [CrossRef]
144. Locht, C.; Coutte, L.; Mielcarek, N. The ins and outs of pertussis toxin. FEBS J. 2011, 278, 4668–4682. [CrossRef] [PubMed]
145. Sanchez, J.; Holmgren, J. Cholera toxin structure, gene regulation and pathophysiological and immunological aspects. Cell. Mol. Life Sci. 2008, 65, 1347–1360. [CrossRef] [PubMed]
146. Collier, R.J. Understanding the mode of action of diphtheria toxin: A perspective on progress during the 20th century. Toxicon 2001, 39, 1793–1803. [CrossRef]
147. El-Kenawi, A.E.; El-Remessy, A.B. Angiogenesis inhibitors in cancer therapy: Mechanistic perspective on classification and treatment rationales. Br. J. Pharmacol. 2013, 170, 712–729. [CrossRef]
148. Kusaka, M.; Sudo, K.; Fujita, T.; Marui, S.; Itoh, F.; Ingber, D.; Folkman, J. Potent anti-angiogenic action of AGM-1470: Comparison to the fumagillin parent. Biochem. Biophys. Res. Commun. 1991, 174, 1070–1076. [CrossRef]
149. Chun, E.; Han, C.K.; Yoon, J.H.; Sim, T.B.; Kim, Y.K.; Lee, K.Y. Novel inhibitors targeted to methionine aminopeptidase 2 (MetAP2) strongly inhibit the growth of cancers in xenografted nude model. Int. J. Cancer 2005, 114, 124–130. [CrossRef]
150. Kidoikhammouan, S.; Seubwai, W.; Silsirivanit, A.; Wongkham, S.; Sawanyawisuth, K.; Wongkham, C. Blocking of methionine aminopeptidase-2 by TNP-470 induces apoptosis and increases chemosensitivity of cholangiocarcinoma. J. Cancer Res. Ther. 2019, 15, 148–152. [CrossRef] [PubMed]
151. Niwano, M.; Arii, S.; Mori, A.; Ishigami, S.; Harada, T.; Mise, M.; Furutani, M.; Fujioka, M.; Imamura, M. Inhibition of tumor growth and microvascular angiogenesis by the potent angiogenesis inhibitor, TNP-470, in rats. Surg. Today 1998, 28, 915–922. [CrossRef] [PubMed]
152. Mauriz, J.L.; Gonzalez, P.; Durán, M.C.; Molpeceres, V.; Culebras, J.M.; Gonzalez-Gallego, J. Cell-cycle inhibition by TNP-470 in an in vivo model of hepatocarcinoma is mediated by a p53 and p21WAF1/CIP1 mechanism. Transl. Res. 2007, 149, 46–53. [CrossRef]
153. Bhargava, P.; Marshall, J.L.; Rizvi, N.; Dahut, W.; Yoe, J.; Figuera, M.; Phipps, K.; Ong, V.S.; Kato, A.; Hawkins, M.J. A Phase I and pharmacokinetic study of TNP-470 administered weekly to patients with advanced cancer. Clin. Cancer Res. 1999, 5, 1989–1995.
154. Audemard, A.; Le Bellec, M.L.; Carluer, L.; Dargère, S.; Verdon, R.; Castrale, C.; Lobbedez, T.; Hurault de Ligny, B. Fumagillin-induced aseptic meningoencephalitis in a kidney transplant recipient with microsporidiosis. Transpl. Infect. Dis. 2012, 14, 147–149. [CrossRef]
155. Molina, J.M.; Goguel, J.; Sarfati, C.; Chastang, C.; Desportes-Livage, I.; Michiels, J.F.; Maslo, C.; Katlama, C.; Cotte, L.; Leport, C.; et al. Potential efficiency of fumagillin in intestinal microsporidiosis due to Enterocytozoon bieneusi in patients with HIV Infection: Results of a drug screening study. AIDS 1997, 11, 1603–1610. [CrossRef]
156. Hou, L.; Mori, D.; Takase, Y.; Meihua, P.; Kai, K.; Tokunaga, O. Fumagillin inhibits colorectal cancer growth and metastasis in mice: In vivo and in vitro study of anti-angiogenesis. Pathol. Int. 2009, 59, 448–461. [CrossRef]
157. Tanaka, S.; Arii, S. Current status and perspective of antiangiogenic therapy for cancer: Hepatocellular carcinoma. Int. J. Clin. Oncol. 2006, 11, 82–89. [CrossRef]
158. Ogawa, H.; Sato, Y.; Kondo, M.; Takahashi, N.; Oshima, T.; Sasaki, F.; Une, Y.; Nishihira, J.; Todo, S. Combined treatment with TNP-470 and 5-fluorouracil effectively inhibits growth of murine colon cancer cells in vitro and liver metastasis in vivo. Oncol. Rep. 2000, 7, 467–472. [CrossRef]
159. Wang, J.; Sheppard, G.S.; Lou, P.; Kawai, M.; BaMaung, N.; Erickson, S.A.; Tucker-Garcia, L.; Park, C.; Bouska, J.; Wang, Y.C.; et al. Tumor suppression by a rationally designed reversible inhibitor of methionine aminopeptidase-2. Cancer Res. 2003, 63, 7861–7869.
160. Hotz, H.G.; Reber, J.A.; Hotz, B.; Sanghavi, P.C.; Yu, T.; Foitzik, T.; Buhr, H.J.; Hines, O.J. Angiogenesis inhibitor TNP-470 reduces human pancreatic cancer growth. J. Gastrointest. Surg. 2001, 5, 131–138. [CrossRef]
161. Kawarada, Y.; Ishikura, H.; Kishimoto, T.; Saito, K.; Takahashi, T.; Kato, H.; Yoshiki, T. Inhibitory effects of the antiangiogenic agent TNP-470 on establishment and growth of hematogenous metastasis of human pancreatic carcinoma in SCID beige mice in vivo. Pancreas 1997, 15, 251–257. [CrossRef]
162. Shishido, T.; Yasoshima, T.; Denno, R.; Mukaiya, M.; Sato, N.; Hirata, K. Inhibition of liver metastasis of human pancreatic carcinoma by angiogenesis inhibitor TNP-470 in combination with cisplatin. Jpn. J. Cancer Res. 1998, 89, 963–969. [CrossRef]
163. Kato, H.; Ishikura, H.; Kawarada, Y.; Furuya, M.; Kondo, S.; Kato, H.; Yoshiki, T. Anti-angiogenic treatment for peritoneal dissemination of pancreas adenocarcinoma: A study using TNP-470. Jpn. J. Cancer Res. 2001, 92, 67–73. [CrossRef]
164. Miyazaki, J.; Tsuzuki, Y.; Matsuzaki, K.; Hokari, R.; Okada, Y.; Kawaguchi, A.; Nagao, S.; Itoh, K.; Miura, S. Combination therapy with tumor-lysate pulsed dendritic cells and antiangiogenic drug TNP-470 for mouse pancreatic cancer. Int. J. Cancer 2005, 117, 499–505. [CrossRef]
165. Ho, D.H.; Wong, R.H. TNP-470 skews DC differentiation to Th1-stimulatory phenotypes and can serve as a novel adjuvant in a cancer vaccine. Blood Adv. 2018, 2, 1664–1679. [CrossRef]
166. Yamaoka, M.; Yamamoto, T.; Masaki, T.; Ikeyama, S.; Sudo, K.; Fujita, T. Angiogenesis inhibitor TNP-470 (AGM-1470) potently inhibits the tumor growth of hormone-independent human breast and prostate carcinoma cell lines. Cancer Res. 2003, 53, 5233–5236.
167. Tucker, L.A.; Zhang, Q.; Sheppard, G.S.; Jiang, F.; McKeegan, E.; Lesniewski, R.; Davidsen, S.K.; Bell, R.L.; Wang, J. Ectopic expression of methionine aminopeptidase-2 causes cell transformation and stimulates proliferation. Oncogene 2008, 27, 3967–3976. [CrossRef]
168. Logothetis, C.J.; Wu, K.K.; Finn, L.D.; Figg, W.; Ghaddar, H.; Gutterman, J.U. Phase I trial of the angiogenesis inhibitor TNP-470 for progressive androgen-independent prostate cancer. Clin. Cancer Res. 2001, 7, 1198–1203.
169. Bo, H.; Ghazizadeh, M.; Shimizu, H.; Kurihara, Y.; Egawa, S.; Moriyama, Y.; Tajiri, T.; Kawanami, O. Effect of ionizing irradiation on human esophageal cancer cell lines by cDNA microarray gene expression analysis. J. Nippon Med. Sch. 2004, 71, 172–180. [CrossRef]
170. Conteas, C.N.; Berlin, O.G.; Ash, L.R.; Pruthi, J.S. Therapy for human gastrointestinal microsporidiosis. Am. J. Trop. Med. Hyg. 2000, 63, 121–127. [CrossRef]
171. Champion, L.; Durrbach, A.; Lang, P.; Delahousse, M.; Chauvet, C.; Sarfati, C.; Glotz, D.; Molina, J.M.Fumagillin for treatment of intestinal microsporidiosis in renal transplant recipients. Am. J. Transpl. 2010, 10, 1925–1930. [CrossRef]
172. Bukreyeva, I.; Angoulvant, A.; Bendib, I.; Gagnard, J.C.; Bourhis, J.H.; Dargère, S.; Bonhomme, J.; Thellier, M.; Gachot, B.; Wyplosz, B. Enterocytozoon bieneusi microsporidiosis in stem cell transplant recipients treated with fumagillin. Emerg. Infect. Dis. 2017, 23, 1039–1041. [CrossRef]
173. Bailey, L.; Ball, B.V. Honey Bee Pathology, 2nd ed.; Academic Press: London, UK, 1991; pp. 1–298.
174. Fries, I. Nosema apis—A parasite in the honey bee colony. Bee World 1993, 74, 5–19. [CrossRef]
175. Bailey, L. Effect of fumagillin upon Nosema apis (Zander). Nature 1953, 171, 212–213. [CrossRef]
176. Katznelson, H.; Jamieson, C.A. Control of Nosema disease of honeybees with fumagillin. Science 1952, 115, 70–71. [CrossRef]
177. Hartwig, A.; Przele¸cka, A. Nucleic acids in the intestine of Apis mellifera infected with Nosema apis and treated with fumagillin DCH: Cytochemical and autoradiographic studies. J. Invertebr. Pathol. 1971, 18, 331–336. [CrossRef]
178. Webster, T.C. Fumagillin affects Nosema apis and honey bees (Hymenopterai Apidae). J. Econ. Entomol. 1994, 87, 601–604. [CrossRef]
179. Williams, G.R.; Sampson, M.A.; Shutler, D.; Rogers, R.E.L. Does fumagillin control the recently detected invasive parasite Nosema ceranae in Western honey bees (Apis mellifera)? J. Invertebr. Pathol. 2008, 99, 342–344. [CrossRef]
180. Huang, W.F.; Solter, L.F.; Yau, P.M.; Imai, B.S. Nosema ceranae escapes fumagillin control in honey bees. PLoS Pathol. 2013, 9. [CrossRef]
181. Zhang, Y.; Li, X.Q.; Li, H.M.; Zhang, Q.H.; Gao, Y.; Li, X.J. Antibiotic residues in honey: A review on analytical methods by liquid chromatography tandem mass spectrometry. Trends Anal. Chem. 2019, 110, 344–356. [CrossRef]
182. Zhang, P.; Nicholson, D.E.; Bujnicki, J.M.; Su, X.; Brendle, J.J.; Ferdig, M.; Kyle, D.E.; Milhous, W.K.; Chiang, P.K. Angiogenesis inhibitors specific for methionine aminopeptidase 2 as drugs for malaria and leishmaniasis. J. Biomed. Sci. 2002, 9, 34–40. [CrossRef]
183. Chen, X.; Xie, S.; Bhat, S.; Kumar, N.; Shapiro, T.A.; Liu, J.O. Fumagillin and fumarranol interact with P. falciparum methionine aminopeptidase 2 and inhibit malaria parasite growth in vitro and in vivo. Chem. Biol 2009, 16, 193–202. [CrossRef]
184. Hillmann, F.; Novohradská, S.; Mattern, D.J.; Forberger, T.; Heinekamp, T.; Westermann, M.; Winckler, T.; Brakhage, A.A. Virulence determinants of the human pathogenic fungus Aspergillus fumigatus protect against soil amoeba predation. Environ. Microbiol. 2015, 17, 2858–2869. [CrossRef]
185. Watanabe, N.; Nishihara, Y.; Yamaguchi, T.; Koito, A.; Miyoshi, H.; Kakeya, H.; Osada, H. Fumagillin suppresses HIV-1 infection of macrophages through the inhibition of Vpr activity. FEBS Lett. 2006, 580, 2598–2602. [CrossRef]
186. Cao, Y. Angiogenesis modulates adipogenesis and obesity. J. Clin. Investig. 2007, 117, 2362–2368. [CrossRef]
187. Lijnen, H.R.; Frederix, L.; Van Hoef, B. Fumagillin reduces adipose tissue formation in murine models of nutritionally induced obesity. Obesity 2010, 18, 2241–2246. [CrossRef]
188. Scroyen, I.; Christiaens, V.; Lijnen, H.R. Effect of fumagillin on adipocyte differentiation and adipogenesis. Biochim. Biophys. Acta 2010, 1800, 425–429. [CrossRef]

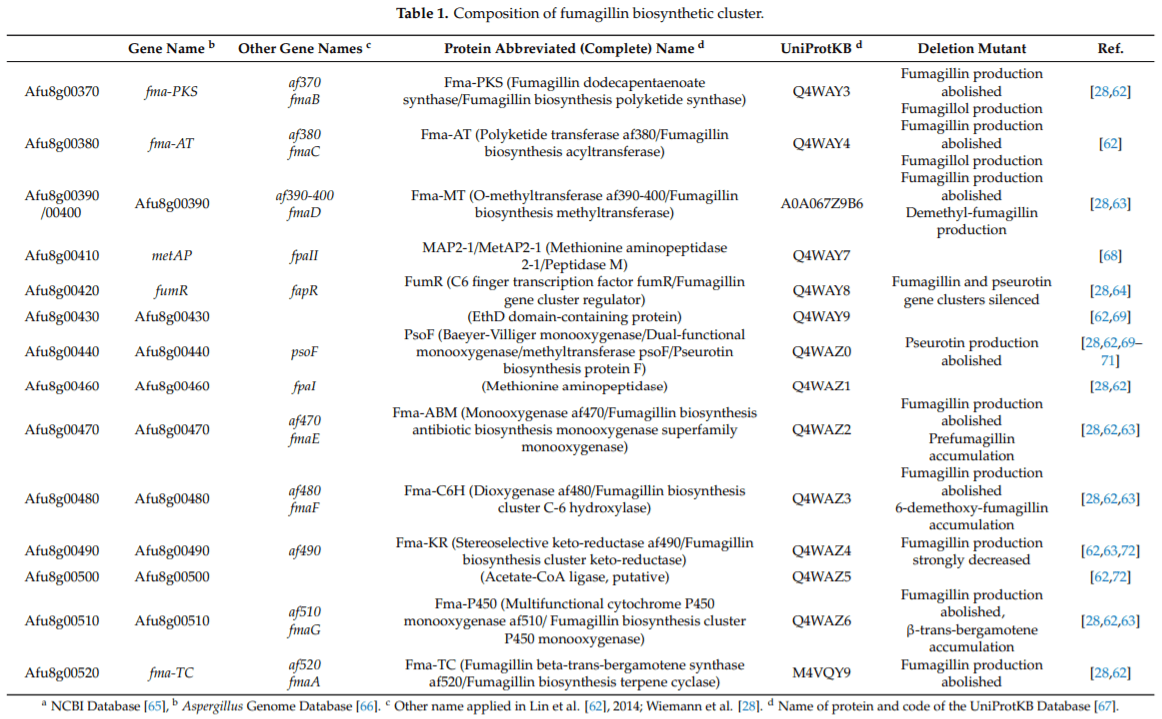







.jpg&w=3840&q=75)