Masked mycotoxins: does breeding for enhanced Fusarium head blight resistance result in more deoxynivalenol-3-glucoside in new wheat varieties?
From economic and environmental points of view, enhancing resistance to Fusarium head blight (FHB) in wheat is regarded as the best option to reduce fungal colonisation and the concomitant mycotoxin contamination. This review focuses on the effect of FHB resistance on deoxynivalenol (DON) and the masked metabolite deoxynivalenol- 3-glucoside (DON-3-glucoside) in wheat. Based on published information complemented with our own results we draw the following conclusions: (1) All investigated wheat cultivars can convert DON to DON-3-glucoside. Hence, detoxification of DON to DON-3-glucoside is not a new trait introduced by recent resistance breeding against FHB.
(2) The amount of DON-3-glucoside relative to DON contamination can be substantial (up to 35%) and is among other things dependent on genetic and environmental factors. (3) Correlation analyses showed a highly significant relationship between the amount of FHB symptoms and DON contamination: breeding for FHB resistance reduces DON contamination. (4) DON contamination data are highly correlated with DON-3-glucoside concentration data: in other words, reduction of DON content through resistance breeding results in a concomitant reduction in DON-3-glucoside content. (5) The DON-3-glucoside/DON ratio increases with decreasing DON contamination: the most resistant lines with the lowest DON contamination show the highest relative level of DON-3-glucoside to DON. In summary, introgressing FHB resistance reduces both DON and DON-3-glucoside levels in the grain, but the reduction is lower for the masked toxin. DON-3-glucoside can represent a possible hazard to human and animal health, especially in wheat samples contaminated with DON close to permitted limits.
Keywords: cereals, trichothecene, Gibberella, ratio masked toxin.
1. Introduction
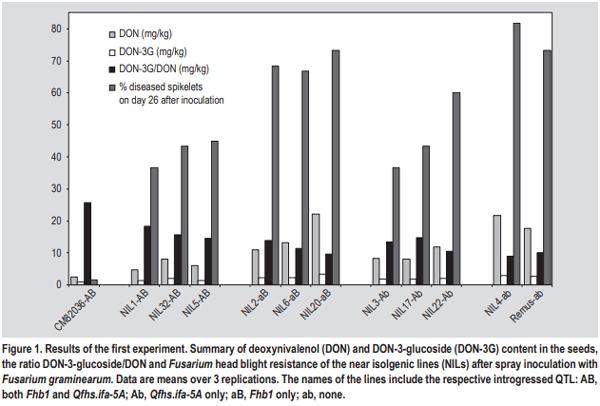
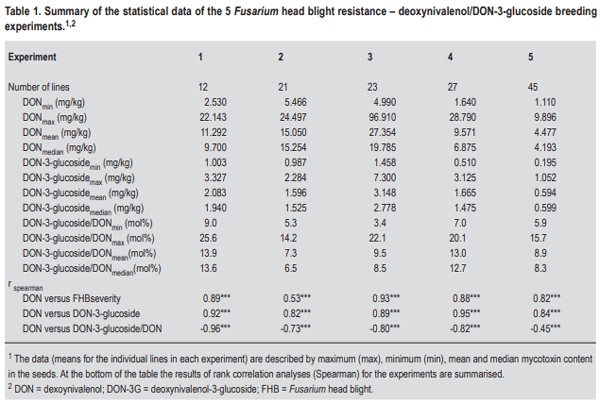
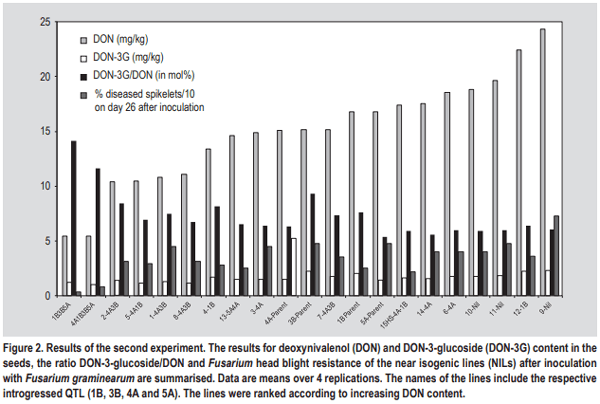
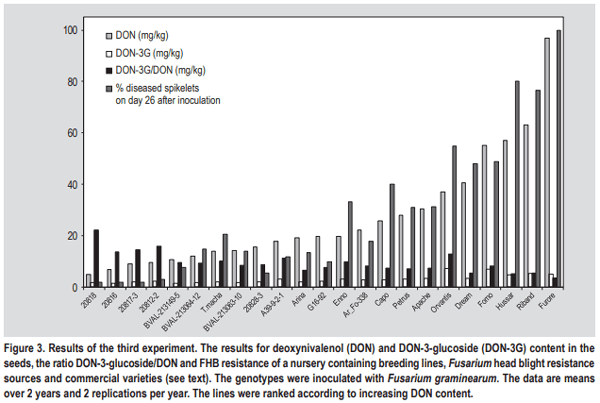
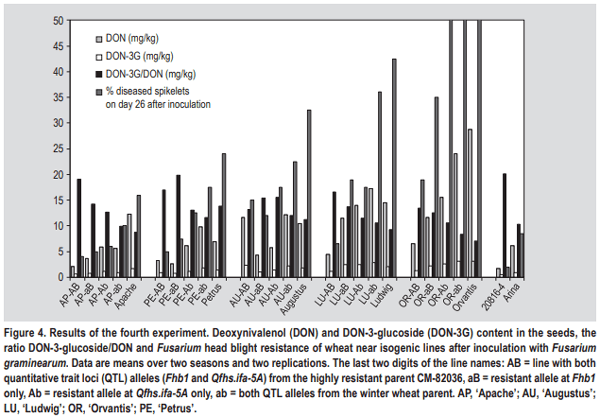
- The simplest explanation for a higher DON-3-glucoside to DON ratio could be the fact that in resistant varieties the fraction of healthy, metabolically active plant tissue remains higher, thus contributing more and/or for a longer period of time to the detoxification of DON. The translocation of DON through the xylem and phloem has been reported, resulting in detectable amounts of toxin also in those kernels that did not show any fungal infection (Kang and Buchenauer, 1999; Pritsch et al., 2001). We speculate that in active plant cells over time more DON can be metabolised to DON-3-glucoside resulting in a shift towards a higher DON-3-glucoside/ DON ratio. Any type of resistance QTL or pyramiding of different QTL resulting in a reduction of FHB damage to the head tissue could contribute to this shift. On the other hand, local DON concentrations in susceptible lines could be so high that the detoxification enzymes (both glucosylation and glutathione pathway (see below)) gets saturated followed by fast dying of the plant tissue. In such tissues all plant conversions of DON will come to a halt and this could result in a comparatively lower DON-3-glucoside to DON ratio.
- A second possibility is that in the scope of co-evolution between host plant (e.g. wheat) and pathogen, mechanisms developed in planta that detoxify DON. DON is a non-host specific toxin and it is a fungal virulence factor. Conjugation is known to be a detoxification process: DON-3-glucoside showed a strongly reduced ability to inhibit protein synthesis of wheat ribosomes compared to DON (Poppenberger et al., 2003). The QTL on chromosome 3B (Fhb1) protects wheat against the phytotoxic effect of DON (Lemmens et al., 2005). It was suggested that Fhb1 is involved in a faster detoxification of DON. Data from Cirlini et al. (2014) showed that ‘Sumai-3’ – a highly FHB resistant variety carrying Fhb1 – detoxifies DON to DON-3- glucoside at a faster rate than susceptible durum lines: as soon as 24 h after inoculation ‘Sumai-3’ showed the highest conversion rate (25 versus 5-6% for the durum lines) and its detoxification ability was confirmed as very high at the time of harvest (84%). Kluger et al. (2015) reported that after application of DON in wheat ears, the mycotoxin can be detoxified via two major pathways: either glucosylation or via the glutathione (GSH) pathway. Fhb1 enhances and intensifies the detoxification via the glucosylation pathway. Wheat cultivars carrying the resistance QTL Fhb1 showed similar metabolism kinetics: formation of DON-3-glucoside was faster, while DON-GSH production was less efficient compared to cultivars which lacked Fhb1. Moreover, all wheat lines harbouring Fhb1 showed significantly elevated DON-3- glucoside/DON abundance ratios (Kluger et al., 2015).
- A combination of 1 and 2.
- All wheat lines that we investigated, even if not specifically bread for FHB resistance, can metabolise DON to DON3-glucoside. Hence this plant detoxification pathway is not a new trait introduced via recent resistance breeding. Still, some specific QTL (e.g. Fhb1) possibly enhance the speed or rate of DON detoxification.
- Combining several QTL for FHB resistance decreases FHB symptom severity.
- DON-3-glucoside content is in general lower than the DON content in the grains (DON-3-glucoside/DON ratio usually <35%).
- Increasing FHB resistance results in a decrease in the absolute contamination of both DON and the masked toxin DON-3-glucoside in the grain.
- An increase in FHB resistance together with a decrease in DON content results in a concomitant increase in the relative fraction of the masked toxin DON-3-glucoside compared to DON.
- This is due to the fact that upon increasing FHB resistance, the reduction of DON content is more efficient than the reduction of the DON-3-glucoside contamination.
- As far as we can judge it, every FHB resistance factor (QTL) or combination of different QTL that significantly reduce DON content shows this effect (increasing DON3-glucoside/DON ratio) in the phenotype.
- It is not clear which biochemical/physiological mechanism is responsible for this increase in the DON3-glucoside to DON ratio. Probably a relatively higher proportion of DON is converted in planta to DON-3- glucoside in lines with an improved FHB resistance.
Abramson, D., Clear, R.M. and Nowicki, T.W., 1987. Fusarium species and trichothecene mycotoxins in suspect samples of 1985 Manitoba wheat. Canadian Journal of Plant Science 67: 611-619.
Audenaert, K., De Boevre, M., Vanheule, A., Callewaert, J., Bekaert, B., Höfte, M., De Saeger, S. and Haesaert, G., 2013. Mycotoxin glucosylation in commercial wheat varieties: Impact on resistance to Fusarium graminearum under laboratory and field conditions. Food Control 34: 756-762.
Bai, G. and Shaner, G., 1994. Scab of wheat: prospects for control. Plant Disease 78: 760-766.
Berthiller, F., Crews, C., Dall’Asta, C., De Saeger, S., Haesaert, G., Karlovsky, P., Oswald, I.P., Seefelder, W., Speijers, G. and Stroka, J., 2013. Masked mycotoxins: a review. Molecular Nutrition and Food Research 5: 165-186.
Berthiller, F., Dall’asta, C., Corradini, R., Marchelli, R., Sulyok, M., Krska, R., Adam, G. and Schuhmacher, R., 2009. Occurrence of deoxynivalenol and its 3-β-D-glucoside in wheat and maize. Food Additives and Contaminants Part A 26: 507-511.
Bottalico, A., 1998. Fusarium diseases of cereals: species complex and related mycotoxin profiles, in Europe. Journal of Plant Pathology 80: 85-103.
Bottalico, A. and Perrone, G., 2002. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. European Journal of Plant Pathology 108: 611-624.
Buerstmayr, H., Ban, T. and Anderson, J.A., 2009. QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat: a review. Plant Breeding 128: 1-26.
Buerstmayr, H. and Lemmens, M., 2015. Breeding healthy cereals: genetic improvement of Fusarium resistance and consequences for mycotoxins. World Mycotoxin Journal 8: 591-602.
Buerstmayr, H., Lemmens, M., Grausgruber, H. and Ruckenbauer, P., 1996. Scab resistance of international wheat germplasm. Cereal Research Communications 24: 195-202.
Buerstmayr, H., Lemmens, M., Hartl, L., Doldi, L., Steiner, B., Stierschneider, M. and Ruckenbauer, P., 2002. Molecular mapping of QTLs for Fusarium head blight resistance in spring wheat, I. Resistance to fungal spread (type II resistance). Theoretical and Applied Genetics 104: 84-91.
Buerstmayr, H., Steiner, B., Hartl, L., Griesser, M., Angerer, N., Lengauer, D., Miedaner, T., Schneider, B. and Lemmens, M., 2003. Molecular mapping of QTLs for Fusarium head blight resistance in spring wheat, II. Resistance to fungal penetration and spread. Theoretical and Applied Genetics 107: 503-508.
Burt, C., Steed, A., Gosman, N., Lemmens, M., Bird, N.,·Ramirez- Gonzalez, R., Holdgate, S. and Nicholson, P., 2015. Mapping a type 1 FHB resistance on chromosome 4AS of Triticum macha and deployment in combination with two type 2 resistances. Theoretical and Applied Genetics 128: 1725-1738.
Cirlini, M., Generotti, S., Dall’Erta, A., Lancioni, P., Ferrazzano, G., Massi, A., Galaverna, G. and Dall’Asta, C., 2014. Durum wheat (Triticum durum Desf.) lines show different abilities to form masked mycotoxins under greenhouse conditions. Toxins 6: 81-95.
Dall’Asta, C., Dall’Erta, A., Mantovani, P., Massi, A. and Galaverna, G., 2013. Occurrence of deoxynivalenol and deoxynivalenol-3-glucoside in durum wheat. World Mycotoxin Journal 6: 83-91.
Da Rocha, M.E.B., Da Chagas Oliveira Freire, F., Maia, F.E.F., Guedes,
M.I.F. and Rondina, D., 2014. Mycotoxins and their effects on human and animal health. Food Control 36: 159-165.
De Boevre, M., Di Mavungu, J.D., Landschoot, S., Audenaert, K., Eeckhout, M., Maene, P., Haesaert, G. and De Saeger, S., 2012. Natural occurrence of mycotoxins and their masked forms in food and feed products. World Mycotoxin Journal 5: 207-219.
De Saeger, S. and Van Egmond, H.P., 2012. Special issue: masked mycotoxins foreword. World Mycotoxin Journal 5: 203-206.
Dill-Macky, R., 2003. Inoculation methods and evaluation of Fusarium
head blight resistance in wheat. In: Leonard, K.J. and Bushnell,
W.R. (eds.) Fusarium head blight of wheat and barley. American Phytopathological Society, St. Paul, MN, USA, pp. 184-210.
European Food Safety Authority (EFSA), 2007. Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to deoxynivalenol (DON) as undesirable substance in animal feed, replacing the public opinion 2004. EFSA Journal 73: 1-42.
European Food Safety Authority (EFSA), 2014. Scientific Opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed. EFSA Journal 12: 3916.
Gareis, M., Bauer, J., Thiem, J., Plank, G., Grabley, S. and Gedek, B., 1990. Cleavage of zearalenone-glycoside, a ‘masked’ mycotoxin, during digestion in swine. Journal of Veterinary Medicine, Series B 37: 236-240. Kang, Z. and Buchenauer, H., 1999. Immunocytochemical localization of Fusarium toxins in infected wheat spikes by Fusarium culmorum.
Physiological and Molecular Plant Pathology 55: 275-288. Kluger, B., Bueschl, C., Lemmens, M., Michlmayr, H., Malachova,
A., Koutnik, A., Maloku, I., Berthiller, F., Adam, G., Krska, R. and Schuhmacher, R., 2015. Biotransformation of the mycotoxin deoxynivalenol in Fusarium resistant and susceptible near isogenic wheat lines. PLoS One 10: e0119656.
Lemmens, M., Scholz, U., Berthiller, F., Dall’Asta, C., Koutnik, A., Schuhmacher, R., Adam, G., Buerstmayr, H., Mesterházy, A., Krska,
R. and Ruckenbauer, P., 2005. The ability to detoxify the mycotoxin deoxynivalenol colocalizes with a major quantitative trait locus for Fusarium head blight resistance in wheat. Molecular Plant Microbe Interaction 18: 1318-1324.
Li, F.Q., Wang, W., Ma, J.J., Yu, C.C., Lin, X.H. and Yan, W.X., 2012.
Natural occurrence of masked deoxynivalenol in Chinese wheat and wheat-based products during 2008-2011. World Mycotoxin Journal 5: 221-230.
Liddell, C.M., 2003. Systematics of Fusarium species and allies associated with Fusarium head blight. In: Leonard, K.J. and Bushnell,
W.R. (eds.) Fusarium head blight of wheat and barley. American Phytopathological Society, St. Paul, MN, USA, pp. 35-43.
Malachova, Z., Dzuman, Z., Veprikova, M., Vaclavikova, M., Zachariasova, M. and Hajslova, J., 2011. Deoxynivalenol, deoxynivalenol-3-glucoside and enniatins: the major mycotoxins found in cereal-based products on the Czech market. Journal of Agricultural Food Chemistry 59: 12990-12997.
Marin, S., Ramos, A.J., Cano-Sancho, G. and Sanchis, V., 2013. Mycotoxins: occurrence, toxicology, and exposure assessment. Food and Chemical Toxicology 60: 218-237.
McKendry, A., 2008. Native resistance: an essential building block for accelerating the development of Scab resistant soft red winter wheat. Cereal Research Communications 36: 135-137.
Mesterhazy, A., 1983. Breeding wheat for resistance to Fusarium graminearum and F. culmorum. Zeitschrift für Pflanzenzüchtung 91: 295-311.
Mesterhazy, A., 1995. Types and components of resistance to Fusarium
head blight of wheat. Plant Breeding 114: 377-386.
Mesterhazy, A., Bartok, T., Mirocha, C.G. and Komoroczy, R., 1999. Nature of wheat resistance to Fusarium head blight and the role of deoxynivalenol for breeding. Plant Breeding 118: 97-110.
Nagl, V., Schwartz, H., Krska, R., Moll, W.-D., Knasmüller, S., Ritzmann, M., Adam, G. and Berthiller, F., 2012. Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in rats. Toxicology Letters 213: 367-373.
Nagl, V., Woechtl, B., Schwartz-Zimmermann, H.E., Hennig-Pauka, I., Moll, W.D., Adam, G. and Berthiller, F., 2014. Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in pigs. Toxicology Letters 229: 190-197.
Ovando-Martínez, M., Ozsisli, B., Anderson, J., Whitney, K., Ohm, J.-B. and Simsek, S., 2013. Analysis of deoxynivalenol and deoxynivalenol- 3-glucoside in hard red spring wheat inoculated with Fusarium graminearum. Toxins 5: 2522-2532.
Parry, D.W., Jenkinson, P. and McLeod, L., 1995. Fusarium ear blight (scab) in small grain cereals – a review. Plant Pathology 44: 207-238. Poppenberger, B., Berthiller, F., Lucyshyn, D., Sieberer, T., Schuhmacher, R., Krska, R., Kuchler, K., Glossl, J., Luschnig, C. and Adam, G., 2003. Detoxification of the Fusarium mycotoxin deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. Journal of Biological Chemistry 278: 47905-47914.
Pritsch, C., Vance, C.P., Bushnell, W.R., Somers, D.A., Hohn, T.M. and Muehlbauer, G.J., 2001. Systemic expression of defense response genes in wheat spikes as a response to Fusarium graminearum infection. Physiological and Molecular Plant Pathology 58: 1-12.
Rasmussen, P.H., Nielsen, K.F., Ghorbani, F., Spliid, N.H., Nielsen, G.C. and Jørgensen, L.N., 2012. Occurrence of different trichothecenes and deoxynivalenol-3-β-D-glucoside in naturally and artificially contaminated Danish cereal grains and whole maize plants. Mycotoxin Research 28: 181-190.
Rudd, J.C., Horsley, R.D., McKendry, A.L. and Elias, E.M., 2001. Host plant resistance genes for Fusarium head blight: sources, mechanisms, and utility in conventional breeding systems. Crop Science 41: 620-627.
Rychlik, M., Humpf, H.-U., Marko, D., Dänicke, S., Mally, A., Berthiller, F., Klaffke, H. and Lorenz, N., 2014. Proposal of a comprehensive definition of modified and other forms of mycotoxins including ‘masked’ mycotoxins. Mycotoxin Research 30: 197-205.
Salameh, A., Buerstmayr, M., Steiner, B., Neumayer, A., Lemmens, M. and Buerstmayr, H., 2011. Effects of introgression of two QTL for Fusarium head blight resistance from Asian spring wheat by marker- assisted backcrossing into European winter wheat on Fusarium head blight resistance, yield and quality traits. Molecular Breeding 28: 485-494.
Saur, L., 1991. Recherce de géniteurs de résistance à la fusariose de l’épi causée par Fusarium culmorum chez le blé et les especes voisines. Agronomie 11: 535-541.
Schweiger, W., Steiner, B., Ametz, C., Siegwart, G., Wiesenberger, G., Berthiller, F., Lemmens, M., Jia, H., Adam, G., Muehlbauer, G.J., Kreil,
D.P. and Buerstmayr, H., 2013. Transcriptomic characterization of two major Fusarium resistance quantitative trait loci (QTLs), Fhb1 and Qfhs.ifa-5A, identifies novel candidate genes. Molecular Plant Pathology 14: 772-785.
Skrbic, B., Malachova, A., Zivancev, J., Veprikova, Z. and Hajslová, J., 2011. Fusarium mycotoxins in wheat samples harvested in Serbia: a preliminary survey. Food Control 22: 1261-1267.
Sneller, C.H., Paul, P. and Guttieri, M., 2010. Characterization of resistance to Fusarium head blight in an Eastern US soft red winter wheat population. Crop Science 50: 123-133.
Snijders, C.H.A., 1990. Genetic variation for resistance to Fusarium head blight in bread wheat. Euphytica 50: 171-179.
Snijders, C.H.A. and Van Eeuwijk, F.A., 1991. Genotype × strain interactions for resistance ton Fusarium head blight caused by Fusarium culmorum in winter wheat. Theoretical and Applied Genetics 81: 239-244.
Stepien, L. and Chelkowski, J., 2010. Fusarium head blight of wheat: pathogenic species and their mycotoxins. World Mycotoxin Journal 3: 107-119.
Sulyok, M., Berthiller, F., Krska, R. and Schumacher, R., 2006. Development and validation of a liquid chromatography/tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize. Rapid Communication in Mass Spectrometry 20: 2649-2659.
Sutton, J.C., 1982. Epidemiology of wheat head blight and maize ear rot caused by Fusarium graminearum. Canadian Journal of Plant Pathology 4: 195-209.
Van Eeuwijk, F.A., Mesterhazy, A., Kling, C.I., Ruckenbauer, P., Saur, L., Burstmayr, H., Lemmens, M., Keizer, L.C.P., Maurin, N. and Snijders, C.H.A., 1995. Assessing non-specificity of resistance in wheat to head blight caused by inoculation with European strains of Fusarium culmorum, F. graminearum and F. nivale using a multiplicative model for interaction. Theoretical and Applied Genetics 90: 221-228.
Vanheule, A., Audenaert, K., De Boevre, M., Landschoot, S., Bekaert, B., Munaut, F., Eeckhout, M., Höfte, M., De Saeger, S. and Haesaert, G., 2014. The compositional mosaic of Fusarium species and their mycotoxins in unprocessed cereals, food and feed products in Belgium. International Journal of Food Microbiology 181: 28-36.
Von der Ohe, C., Ebmeyer, E., Korzun, V. and Miedaner, T., 2010. Agronomic and quality performance of winter wheat backcross populations carrying non-adapted Fusarium head blight resistance QTL. Crop Science 50: 2283-2290.
Warth, B., Fruhmann, P., Wiesenberger, G., Kluger, B., Sarkanj, B., Lemmens, M., Hametner, C., Fröhlich, J., Adam, G., Krska, R. and Schuhmacher, R., 2015. Deoxynivalenol-sulfates: identification and quantificationof novel conjugated (masked) mycotoxins in wheat. Analytical and Bioanalytical Chemistry 407: 1033-1039.
Wong, L.S.L., Tekauz, A., Leisle, D., Abramson, D. and McKenzie, R.I.H., 1992. Prevalence, distribution, and importance of fusarium head blight in wheat in Manitoba. Canadian Journal of Plant Pathology 14: 233-238.
Zhang, J.X., Jin, Y., Rudd, J.C. and Bockelman, H.E., 2008. New Fusarium head blight resistant spring wheat germplasm identified in the USDA national small grains collection. Crop Science 48: 223-235.







.jpg&w=3840&q=75)