Long-Term Effects of Ochratoxin A on the Glutathione Redox System and Its Regulation in Chicken
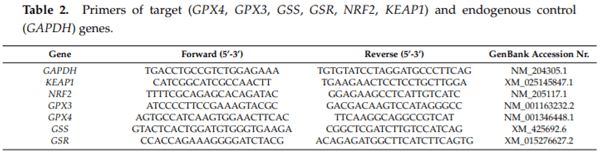
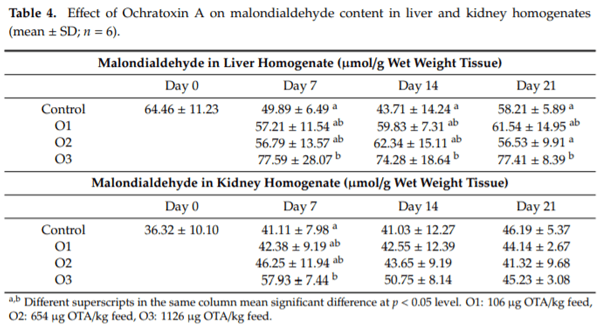
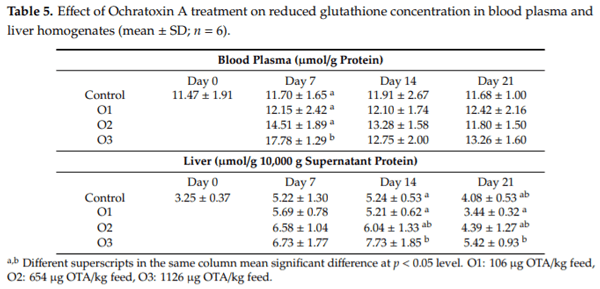
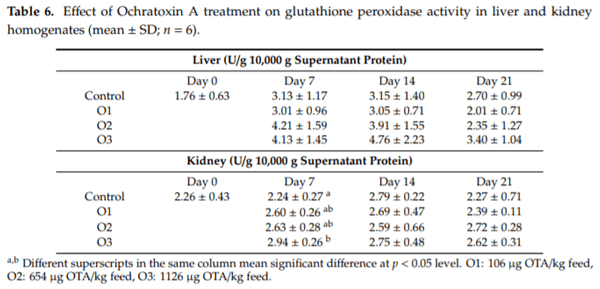
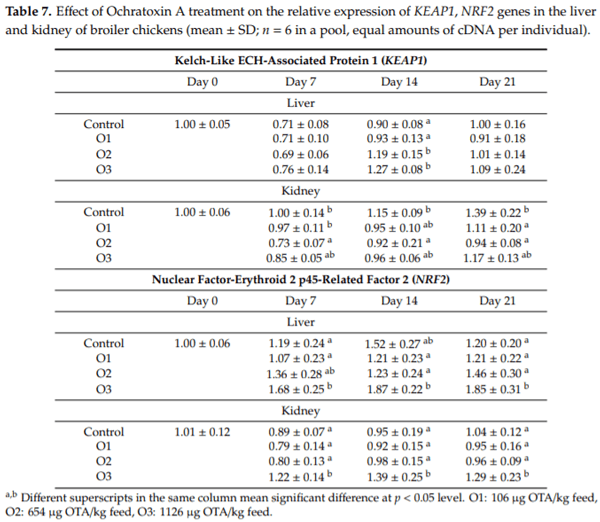
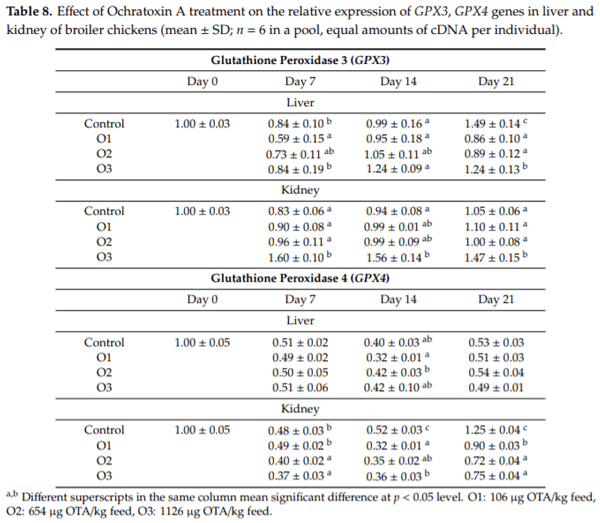
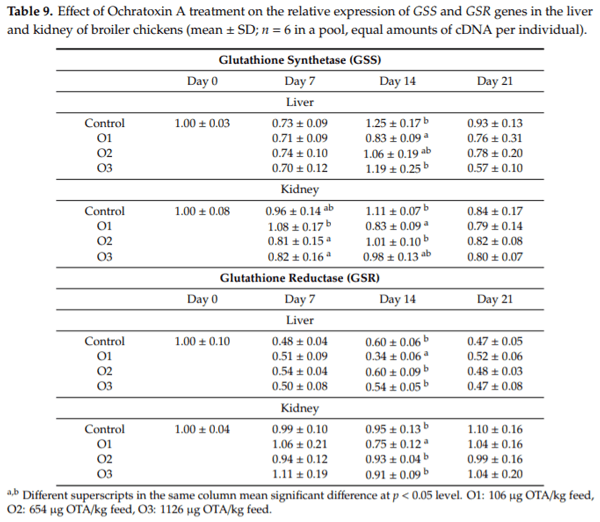
The purpose of this study was to evaluate the effect of three-weeks ochratoxin A (OTA) exposure on some lipid peroxidation parameters, reduced glutathione concentration and glutathione-peroxidase activity, as well as expression of oxidative stress response-related (KEAP1, NRF2) and glutathione system (GPX3, GPX4, GSS, GSR) genes in chickens. Three levels of exposure (106, 654 and 1126 g/kg feed) were applied. The results showed that OTA initiated free radical formation, which was suggested by the increase in the malondialdehyde content in the liver and kidney, which was more marked in the liver, depending on the length of exposure and dose. Reduced glutathione concentration increased as an effect of the highest OTA dose in blood plasma and in liver, but not in red blood cell hemolysates and the kidney. Glutathione peroxidase activity did not change in the blood and showed increasing tendency in the liver, and significant increase in the kidney. Expression of KEAP1 gene showed up-regulation in the liver, and down-regulation in the kidney, but overexpression of NRF2 gene was found in the liver and kidney at the highest dose. However, down-regulation of Nrf2 dependent genes, GPX3, GPX4, GSS and GSR, suggested an improper antioxidant response at the protein level, thus oxidative stress occurred, even at the dose of the EU regulatory limit for poultry diets.
Keywords: Ochratoxin A; glutathione; glutathione-peroxidase; oxidative stress; gene expression.


- Van der Merwe, K.J.; Steyn, P.S.; Fourie, L.; Scott, D.B.; Theron, J.J.; Ochratoxin, A. A toxic metabolite produced by Aspergillus ochraceus Wilh. Nature 1965, 205, 1112–1113. [CrossRef] [PubMed]
- O’Brien, E.; Dietrich, D.R. Ochratoxin A: The continuing enigma. Crit. Rev. Toxicol. 2005, 35, 33–60. [CrossRef] [PubMed]
- Li, S.; Marquardt, R.R.; Frohlich, A.A.; Vitti, T.G.; Crow, G. Pharmacokinetics of ochratoxin A and its metabolites in rats. Toxicol. Appl. Pharmacol. 1997, 145, 82–90. [CrossRef] [PubMed]
- Biomin World Mycotoxin Survey. Annual Report No. 15. 2018. Available online: https://www.biomin.net/en/articles/biomin-world-mycotoxin-survey-report-2018/?utm_source=AAF&utm_medium=Advertorial&utm_campaign=MTXSurvey (accessed on 2 May 2019).
- Dirheimer, G.; Creppy, E.E. Mechanism of action of ochratoxin A. IARC Sci. Publ. 1991, 115, 171–186.
- Huff, W.E.; Wyatt, R.D.; Tucker, T.L.; Hamilton, P.B. Ochratoxicosis in the broiler chicken. Poult. Sci. 1974, 53, 1585–1591. [CrossRef] [PubMed]
- Hu , W.W.; Wyatt, R.D.; Hamilton, P.B. Nephrotoxicity of dietary ochratoxin A in broiler chickens. Appl. Microbiol. 1975, 30, 48–51. [PubMed]
- 8. Solcan, C.; Timofte, D.; Floristean, V.C.; Carter, S.D.; Solcan, G. Ultrastructural lesions and immunochemical analysis of Bcl-2 protein expression in the kidney of chickens with experimental ochratoxicosis. Acta Vet. Hung. 2013, 61, 344–353. [CrossRef]
- Marin-Kuan, M.; Nestler, S.; Verguet, C.; Bezencon, C.; Piguet, D.; Mansourian, R.; Holzwarth, J.; Grigorov, M.; Delatour, T.; Mantle, P.; et al. A toxicogenomics approach to identify new plausible epigenetic mechanisms of ochratoxin a carcinogenicity in rat. Toxicol. Sci. 2006, 89, 120–134. [CrossRef] [PubMed]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Novotna, E. Ochratoxin A: Developmental and reproductive toxicity—An overview. Dev. Reprod. Toxicol. 2013, 98, 493–502. [CrossRef]
- Gupta, S.; Jindal, N.; Khokhar, R.S.; Gupta, A.K.; Ledoux, D.R.; Rottinghaus, G.E. E ect of ochratoxin A on broiler chicks challenged with Salmonella gallinarum. Br. Poult. Sci. 2005, 46, 443–450. [CrossRef]
- Kumar, A.; Jindal, N.; Shukla, C.L.; Pal, Y.; Ledoux, D.R.; Rottinghaus, G.E. E ect of ochratoxin A on Escherichia coli-challenged broiler chicks. Avian Dis. 2003, 47, 415–424. [CrossRef]
- Pozzo, L.; Salamano, G.; Mellia, E.; Gennero, M.S.; Doglione, L.; Cavallarin, L.; Tarantola, M.; Forneris, G.; Schiavone, A. Feeding a diet contaminated with ochratoxin A for chickens at the maximum level recommended by the EU for poultry feeds (0.1 mg/kg). 1. E ects on growth and slaughter performance, haematological and serum traits. J. Anim. Physiol. Anim. Nutr. 2013, 97, 13–22. [CrossRef] [PubMed]
- Gareis, M.; Scheuer, R. Ochratoxin A in meat and meat products. Arch. Lebensm. 2000, 51, 102–104.
- Gentles, A.; Smith, E.E.; Kubena, L.F.; Du us, E.; Johnson, P.; Thompson, J.; Harvey, R.B.; Edrington, T.S. Toxicological evaluations of cyclopiazonic acid and ochratoxin A in broilers. Poult. Sci. 1999, 78, 1380–1384. [CrossRef] [PubMed]
- Luhe, A.; Hildebrand, H.; Bach, U.; Dingermann, T.; Ahr, H.J. A new approach to studying ochratoxin A (OTA)-induced nephrotoxicity: Expression profiling in vivo and in vitro employing cDNA microarrays. Toxicol. Sci. 2003, 73, 315–328. [CrossRef] [PubMed]
- Schaaf, G.J.; Nijmeijer, S.M.; Maas, R.F.; Roestenberg, P.; de Groene, E.M.; Fink-Gremmels, J. The role of oxidative stress in the ochratoxin A-mediated toxicity in proximal tubular cells. Biochim. Biophys. Acta 2002, 1588, 149–158. [CrossRef]
- Hoehler, D.; Marquardt, R.R. Influence of vitamins E and C on the toxic e ects of ochratoxin A and T-2 toxin in chicks. Poult. Sci. 1996, 75, 1508–1515. [CrossRef]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [CrossRef]
- Suzuki, T.; Yamamoto, M. Molecular basis of the Keap1–Nrf2 system. Free Radic. Biol. Med. 2015, 88 Pt B, 93–100. [CrossRef]
- Cavin, C.; Delatour, T.; Marin-Kuan, M.; Holzhauser, D.; Higgins, L.; Bezencon, C.; Guignard, G.; Junod, S.; Richoz-Payot, J.; Gremaud, E.; et al. Reduction in antioxidant defenses may contribute to ochratoxin A toxicity and carcinogenicity. Toxicol. Sci. 2007, 96, 30–39. [CrossRef]
- Boesch-Saadatmandi, C.; Wagner, A.E.; Graeser, A.C.; Hundhausen, C.; Wol ram, S.; Rimbach, G. Ochratoxin A impairs Nrf2-dependent gene expression in porcine kidney tubulus cells. J. Anim. Physiol. Anim. Nutr. 2009, 93, 547–554. [CrossRef] [PubMed]
- Wild, A.C.; Moinova, H.R.; Mulcahy, R.T. Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J. Biol. Chem. 1999, 274, 33627–33636. [CrossRef] [PubMed]
- Motohashi, H.; Yamamoto, M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004, 10, 549–557. [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Cole, R.N.; Itoh, K.; Wakabayashi, N.; Katoh, Y.; Yamamoto, M.; Talalay, P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA 2002, 99, 11908–11913. [CrossRef] [PubMed]
- NCIB (National Center for Biotechnology Information) RefSeq: NCBI Reference Sequence Database. 2014. Available online: http://www.ncbi.nlm.nih.gov/refseq (accessed on 15 April 2019).
- Zeferino, C.P.; Wells, K.D.; Moura, A.S.A.M.T.; Murarolli, R.A.; Rottinghaus, G.E.; Ledoux, D.R. Gene expression in the kidneys of broilers fed ochratoxin A for different time periods. World Mycotox J. 2016, 9, 257–268. [CrossRef]
- Stroka, J.; Ambrosio, M.; Doncheva, I.; Lerda, D.; Mischke, C.; Breidbach, A. Validation of an Analytical Method to Determine the Content of Ochratoxin A in Animal Feed; European Commission Joint Research Centre Institute for Reference Materials and Measurements; O ce for O cial Publications of the European Communities: Luxembourg, 2009; p. 54.
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [PubMed]
- Botsoglou, N.A.; Fletouris, D.J.; Papageorgiou, G.E.; Vassilopoulos, V.N.; Mantis, A.J.; Trakatellis, A.G. Rapid, sensitive and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food and feedstu samples. J. Agric. Food Chem. 1994, 42, 1931–1937. [CrossRef]
- Rahman, I.; Biswas, S.K.; Kode, A. Oxidant and antioxidant balance in the airways and airway diseases. Eur. J. Pharmacol. 2006, 533, 222–239. [CrossRef]
- Lawrence, R.A.; Burk, R.F. Glutathione peroxidase activity in selenium deficient rat liver. Biochem. Biophys. Res. Commun. 1976, 71, 952–956. [CrossRef]
- Weichselbaum, T.E. An accurate and rapid method for the determination of protein in small amounts of serum and plasma. Am. J. Clin. Pathol. 1948, 16, 40–43. [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [PubMed]
- Yarru, L.P.; Settivari, R.S.; Antoniou, E.; Ledoux, D.R.; Rottinghaus, G. Toxicological and gene expression analysis of the impact of aflatoxin B1 on hepatic function of male broiler chicks. Poult. Sci. 2009, 88, 360–371. [CrossRef] [PubMed]
- Salem, R.; El-Habashi, N.; Fadl, S.E.; Sakr, O.A.; Elbialy, Z.I. E ect of probiotic supplement on aflatoxicosis and gene expression in the liver of broiler chicken. Environ. Toxicol. Pharmacol. 2018, 60, 118–127. [CrossRef] [PubMed]
- Erdélyi, M.; Balogh, K.; Pelyhe, C.; Kövesi, B.; Nakade, M.; Zándoki, E.; Mézes, M.; Kovács, B. Changes in the regulation and activity of glutathione redox system, and lipid peroxidation processes in short-term aflatoxin B1 exposure in liver of laying hens. J. Anim. Physiol. Anim. Nutr. 2018, 102, 947–952. [CrossRef] [PubMed]
- Nakade, M.; Pelyhe, C.; Kövesi, B.; Balogh, K.; Kovács, B.; Szabó-Fodor, J.; Zándoki, E.; Mézes, M.; Erdélyi, M. Short-term e ects of T-2 toxin or deoxynivalenol on glutathione status and expression of its regulatory genes in chicken. Acta Vet. Hung. 2018, 66, 28–39. [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [CrossRef] [PubMed]
- Frye, C.E.; Chu, F.S. Distribution of ochratoxin A in chicken tissues and eggs. J. Food Saf. 1977, 1, 147–159. [CrossRef]
- Soyöz, M.; Özçelik, N.; Kihnç, I.; Altunta¸s, I. The e ects of ochratoxin A on lipid peroxidation and antioxidant enzymes: A protective role of melatonin. Cell Biol. Toxicol. 2004, 20, 213–219. [CrossRef]
- Abdel-Wahhab, M.A.; Abdel-Galil, M.M.; El-Lithey, M. Melatonin counteracts oxidative stress in rats fed an ochratoxin A contaminated diet. J. Pineal Res. 2005, 38, 130–135. [CrossRef] [PubMed]
- Omar, R.F.; Hasino , B.B.; Mejilla, F.; Rahimtula, A.D. Mechanism of ochratoxin A stimulated lipid peroxidation. Biochem. Pharmacol. 1990, 40, 1183–1191. [CrossRef]
- Petrik, J.; Zanic-Grubisic, T.; Barisic, K.; Pepeljnjak, S.; Radic, B.; Ferencic, Z.; Cepelak, I. Apoptosis and oxidative stress induced by ochratoxin A in rat kidney. Arc. Toxicol. 2003, 77, 685–693. [CrossRef] [PubMed]
- Tozlovanu, M.; Canadas, D.; Pfohl-Leszkowicz, A.; Frenette, C.; Paugh, R.J.; Manderville, R.A. Glutathione conjugates of ochratoxin A as biomarkers of exposure. Arch. Ind. Hyg. Toksikol. 2012, 63, 417–427. [CrossRef] [PubMed]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sánchez-Pérez, P.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015, 6, 183–197. [CrossRef] [PubMed]
- Avissar, N.; Ornt, D.B.; Yagil, Y.; Horowitz, S.; Watkins, R.H.; Kerl, E.A.; Takahashi, K.; Palmer, I.S.; Cohen, H.J. E ect of renal failure on blood plasma glutathione peroxidase activity in dialysed patients. Am. J. Physiol. 1994, 266, C367–C372. [CrossRef] [PubMed]
- Imai, H.; Nakagawa, Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx,GPx4) in mammalian cells. Free Radic. Biol. Med. 2003, 34, 145–169. [CrossRef]
- Rhee, S.G.; Yang, K.-S.; Kang, S.W.; Woo, H.A.; Chang, T.-S. Controlled elimination of intracellular H2O2: Regulation of peroxiredoxin, catalase, and glutathione peroxidase via post-translational modification. Antioxid. Redox Signal. 2005, 7, 619–626. [CrossRef] [PubMed]
- Franz, C.; Baser, K.H.C.; Windisch, W. Essential oils and aromatic plants in animal feeding—A European perspective. A review. Flavour Fragr. J. 2010, 25, 327–340. [CrossRef]







.jpg&w=3840&q=75)