Evaluation of the ability of Saccharomyces cerevisiae and mannan oliosaccharides to ameliorate the adverse effects of aflatoxin B1 in broiler chickens
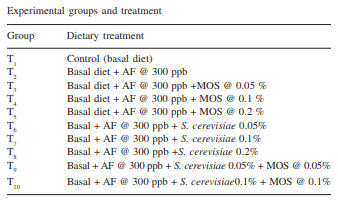
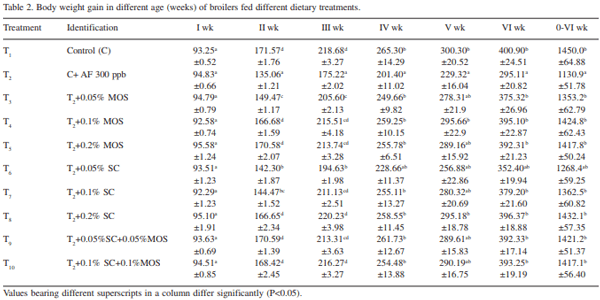
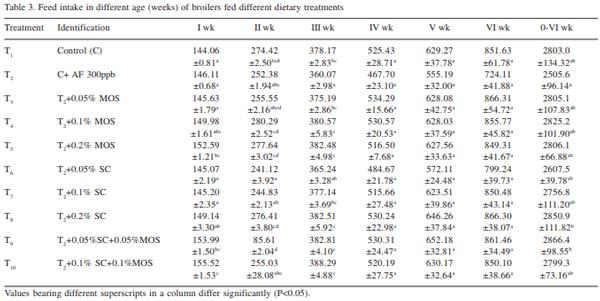
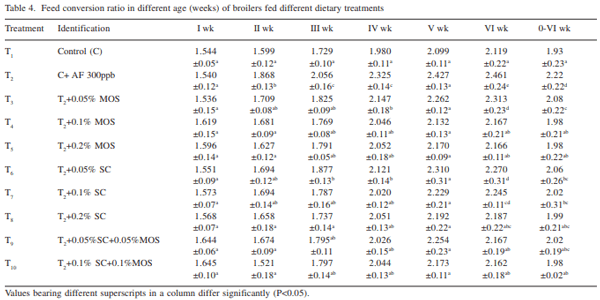
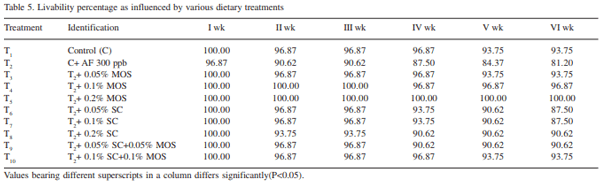
The efficacy of Saccharomyces cerevisiae (SC) and mannan oligosaccharides (MOS) alone or in combinations as aflatoxin binders in the diets of broiler chickens containing 300 ppb aflatoxin B1 was investigated. A total of 320 day-old broiler chicks were divided into 10 treatment groups (T1 - control; T2 - T1 + 300 ppb AFB1 ; T3 - T2 + 0.05% MOS; T4 - T2 + 0.1% MOS; T5 - T2 + 0.2% MOS; T6 - T2 + 0.05% SC; T7 - T2 + 0.1% SC; T8 - T2 + 0.2% SC; T9 - T2 + 0.05% MOS+ 0.05% SC; T10 - T2 + 0.1% MOS + 0.1% SC). Each diet was offered from day-old to 42 days of age to four replicated groups of 8 birds each. During overall growth period (0-6 weeks), the body weight gain (BWG) of broiler in control group (T1 ) was 1450.0 g which significantly (P<0.05) reduced to 1131.0 g in aflatoxin alone fed group (T2 ). The BWG in other treatments (T3 -T10) varied from 1268.4g in T6 to 1432.1 g in T8 and the values recorded were comparable to that of control group. Inclusion of binders in aflatoxin contaminated diet, alone or in combinations, improved (P<0.05) the BWG during 0-6 weeks growth period. The overall feed consumption in all the treatment groups was statistically similar to that of control group, however, the FI in groups T8 and T9 was statistically higher than that of aflatoxin alone fed group (T2 ). During overall growth period, the FCR in control group was 1.932 which significantly (P<0.05) increased to 2.22 due to administration of aflatoxin. The overall FCR in groups T3 , T6 and T7 was statistically higher than that of control group. This indicated that supplementation of MOS at 0.05% and SC at 0.05 and 0.1% level to the aflatoxin contaminated diet may not be sufficient to curb the harmful effect of aflatoxicosis in broiler diet. Addition of MOS and SC (alone or in combination) improved the livability percentage in broiler chickens. MOS at 0.1 and 0.2% level of inclusion was more effective than their SC counterpart in improving the livability percentage in broiler chickens. It is concluded that aflatoxin in feed at 300 ppb level impaired the performance in the terms of body weight gain, feed intake, feed efficiency and livability in broiler chickens during period of 0- 6 weeks. Use of binders MOS and SC (at the rate 0.05%, 0.1%, 0.2%) and their combination ameliorated the effect of aflatoxin partially or completely in dose-dependent manner. The 0.2% level of MOS and SC is more effective than 0.05% and 0.1% level in counteracting the 300 ppb of aflatoxin in the feed.
Key words: Aflatoxin, broiler chicken, binder, Saccharomyces cerevisiae, mannan oligosaccharides.
AOAC. 1990. Official Methods of Analysis. 15th edn. Association of Official Analytical Chemists, Washington, DC.
Coulombe, Jr.R.A., Guarisco, J.A., Klein, P.J. and Hall, J.O. 2005. Chemoprevention of aflatoxicosis in poultry by dietary butylated hydroxytoluene. Animal Feed Science and Technology, 121: 217-25.
Denli, M., Blandon, J.C., Guynot, M.E., Salado, S. and Perez, J.F. 2009. Effects of dietary Aflatoxin on performance, serum biochemistry, histopathological changes and aflatoxin residues in broilers exposed to aflatoxin B1 . Poultry Science, 88: 1444-51.
Duncan, D.B. 1955. Multiple range and multiple F tests. Biometrics, 11: 1-42.
Galvano, F., Piva, A., Ritieni, A. and Galvano, G. 2001. Dietary strategies to counteract the effects of mycotoxins: A review. Journal of Food Protection, 64: 120-31.
Gopi, K. 2006. Influence of melatonin on aflatoxicosis in broiler chickens. M.V.Sc. thesis, Deemed University, IVRI, Izatnagar, India.
Mahesh, B.K. and Devegowda, G. 1996. Ability of aflatoxin binders to bind aflatoxin in contaminated poultry feed-an in vitro study. Proceedings of the 20th Worlds Poultry Congress, New Delhi, 4, 296.
Miazzo, R., Rosa, C.A.R., Dequeiroz-Carvalho, E.C., Mangoli, C., Chiacchiera, S.M., Palacio, G., Saenz, M., Kikot, A., Basaldella, E. and Dalcero, A. 2000. Efficacy of synthetic zeolite to reduce the toxicity of aflatoxin in broiler chicks. Poultry Science, 79: 1-6.
Modirsanei, M., Khosravi, A.R., Kiaei, S.M.M., Bozorgmehri Fard, M.H., Gharagozloo, M.J. and Khazraeinia, P. 2004. Efficacy of Dietary Natural Zeolite and Saccharomyces cerevisiae in Counteracting Aflatoxicosis in Broiler Chicks, Journal of Applied Animal Research, 26: 39-44
Oguz, H., Hadimli, H.H., Kurtoglu, V. and Erganis, O. 2003. Evaluation of humoral immunity of broilers during chronic aflatoxin (50 and 100 ppb) and clinoptilolite exposure. Revue de Medicine Veterinair,. 154: 483–86.
Oguz, H., Kececi, T., Birdane, Y.O., Onder, F. and Kurtoglu, V. 2000. Effect of clinoptilolite on serum biochemical and haematological characters of broiler chickens during experimental aflatoxicosis. Research in Veterinary Science, 69: 89-93.
Oguz H. and Parlat S.S. 2004. Effects of dietary mannanoligosaccharide on performance of Japanese quail affected by aflatoxicosis. South African Journal of Animal Science, 34: 144–48.
Ortatatli, M. and Oguz, H. 2001. Ameliorative effects of dietary clinoptilolite on pathological changes in broiler chickens during aflatoxicosis. Research in Veterinary Science, 71: 59-66.
Parlat, S.S., Ozcan, M. and Oguz, H., 2001. Biological suppression of aflatoxicosis in Japanese quail (Coturnix coturnix japonica) by dietary addition of yeast (Saccharomyces cerevisiae). Research in Veterinary Science, 71: 207-11.
Pons, D., Cucullu, A.P., Lee, L.S., Robertson, J.A. and Goldblatt, L.A. 1966. Determination of aflatoxins in agricultural products: Use of aqueous acetone for extraction. Journal of Analytical Chemistry, 49: 544-52.
Raju, M.V.L.N. and Devegowda, G. 2000. Influence of esterified glucomannan on performance and organ morphology, serum biochemistry and hematology in broilers exposed to individual and combined mycotoxicosis (aflatoxin, ochratoxin and T-2 toxin). British Poultry Science, 41: 640-50.
Sapocota, D., Islam, R. and Baruah K.K. 2007. Protective efficacy of dietary methionine in experimental aflatoxicosis in broilers. Indian Journal of Animal Science, 77: 1170-72.
Shotwell, O.L., Hesseltine, C.V., Stubblefield, R.D. and Sorenson, W.G. 1996. Production of aflatoxin on rice. Applied Microbilogy, 14: 425-29.
Silambarasan, S. 2011. Efficacy of Diatomaceous earth, Sodium bentonite and Zeolite as aflatoxin adsorbents in broiler chickens. M.V.Sc. thesis, Deemed University, IVRI, Izatnagar, India. Singh, R., Shrivastav, H.P. and Shrivastav, A.K., 2010. Mycotoxin contamination in maize as poultry feed. Indian Journal of Poultry Science, 45: 108-10.
Snedecor, G.W. and Cochran, W.G. 1980. Statistical Methods. 6th edn. Iowa State Univ Press, Ames, Iowa. Stanley, V.G., Ojo, R., Woldensenbet, S. and Hutchinson, D.H., 1993. The use of Saccharomyces cerevisiae to suppress the effect of aflatoxicosis in broiler chicks. Poultry Science, 72: 1867-72
Suksombat, W., Suksombat, P. and Mirattanaphrai, R. 2011. Effect of Commercial or Bovine Yeasts on the Performance and Blood Variables of Broiler Chickens Intoxicated with Aflatoxins. World Academy of Science, Engineering and Technology, 58: 664-68
Talapatra, S.K., Ray, S.C. and Sen, K.C. 1940. Estimation of phosphorus, choline, calcium, magnesium, sodium and potassium in feeding stuffs. Journal of Veterinary Science and Animal Husbandary, 10: 243-45.
Zhao, J., Shirley, R.B., Dibner, J.D., Uraizee, F., Officer, M., Kitchell, M., Vazquez-Anon, M. and Knight, C.D. 2010. Comparison of hydrated sodium calcium aluminosilicate and yeast cell wall on counteracting aflatoxicosis in broiler chicks. Poultry Science, 89: 2147–56.
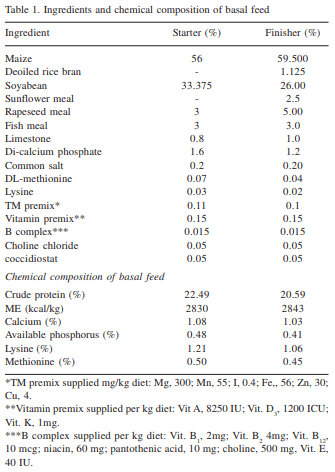
Saccharomyces cerevisiae (SC) has considerable binding capacity for aflatoxin (Galvano et al., 2001). Mannan oligosaccharides (MOS) derived from the cell wall of Saccharomyces cerevisiae was also reported to have high binding capacity (95%) for aflatoxin (Mahesh and Devegowda, 1996). The present investigation was undertaken to test the efficacy of SC and MOS in alleviating aflatoxicosis in broiler chickens. This work was carried out in my laboratory and is the original piece of research work undertaken in the project "Management of Mycotoxicosis in Poultry".
Dr. Ram Singh Bibyan.










