Amelioration of Aflatoxicosis in Coloured Broiler Chickens by Dietary Butylated Hydroxytoluene
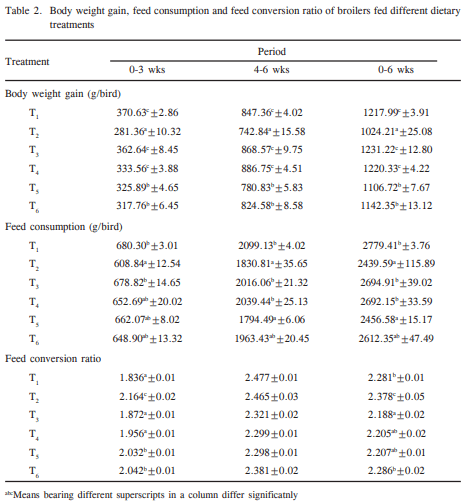
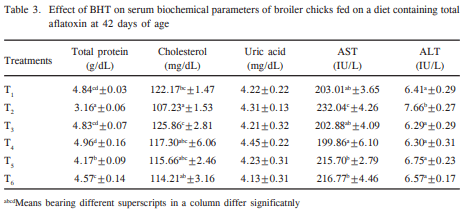
The effect of butylated hydroxytoluene (BHT) on aflatoxicosis in coloured broiler chickens was investigated at two levels (1000 and 2000 ppm) in diet containing 1.0 ppm total aflatoxin (AF: 76.45% AFB1, 10.52% AFB2, 9.89% AFG1 and 3.14% AFG2). A total of 144 day-old broiler chicks were divided into six treatment groups (T1 control, T2 1.0 ppm AF, T3 1000 ppm BHT, T4 2000 ppm BHT, T5 1.0 ppm AF+1000 ppm BHT, and T6 1.0 ppm AF+2000 ppm BHT). Each diet was fed to four replicated groups of 8 birds each from day 1 to 42 days of age. Significant (P<0.05) reduction in BW gain was recorded in T2, T5 and T6 as compared to control, and the depression in BW gain was evident from 2nd week of age. There was significant (P<0.05) improvement of BW gain on supplementation of BHT in diet with AF. Feed consumption in T2 was significantly (P<0.05) less compared to T1, whereas it improved partially in T5 and T6. Aflatoxin treatment also deteriorated the feed efficiency, however, significant (P<0.05) improvement in feed efficiency was observed in T5 and T6. The serum protein concentration in T2 was the lowest as compared to other treatment groups. Cholesterol content in T1 was significantly (P<0.05) higher than that of T2 and comparable to other dietary treatments. Uric acid content did not differ significantly (P<0.05) among various treatment groups. The BHT treatment significantly (P<0.05) improved the serum and cholesterol concentration over AF treatment. The aspartate- and alanine- aminotransferase activities in T1 were significantly (P<0.05) lower than that of T2 and remained almost comparable to T3, T4, T5 and T6. The results showed that BHT inclusion in the diet at 1000 and 2000 ppm provided moderate protection against the adverse effects of aflatoxicosis in terms of investigated parameters.
Key words: Broiler, Aflatoxin, Butylated hydroxytoluene, Feed efficiency.
INTRODUCTION
Abo-Norag, M., Edrington, T.S., Kubena, L.F. and Harvey, R.B. 1995. Influence of hydrated sodium calcium aluminosilicate and virginiamycin on aflatoxicosis in broiler chicks. Poultry Science, 74: 626-32.
Amer, A.M.M., Fahim, E.M.M. and Ibrahim, R.K. 1998. Effect of aflatoxicosis on the kinetic behavior of ceftiofur in chickens. Research in Veterinary Science, 65: 115- 118.
Azam, A.H. and Gabal, M.A. 1997. Interaction of aflatoxin in feed and immunization against selected infectious disease. Avian Pathology, 26: 317- 325.
Bailey, C.A., Latimer, G.W., Barr, A.C., Wigle, W.L., Haq, A.U., Balthrop, J.E. and Kubena, L.F. 2006. Efficacy of montmorillonite (Nova Sil PLUS) for protecting full-term broilers from aflatoxicosis. Journal of Applied Poultry Research, 15: 198- 206.
Basmacioglu, H., Oguz, H., Ergul, M., Col, R. and Birdabe, Y.O. 2005. Effect of dietary esterified glucomannan on performance, serum biochemistry and heamatology in broilers exposed to aflatoxin. Czech Journal of Animal Science, 50: 31-39.
Bintvihok, A. and Kositcharoenkul, S. 2006. Effect of dietary calcium propionate on performance, hepatic enzyme activities and aflatoxin residues in broilers fed a diet containing low levels of aflatoxin B1. Toxicon, 47: 41- 46.
Coulombe, R.A., Guarisco, J.A., Klein, P.J. and Hall, J.O. 2005. Chemoprevention of aflatoxicosis in poultry by dietary butylated hydroxytoluene. Animal Feed Science and Technology, 121: 217-25.
Dalvi, R.R. 1986. An overview of aflatoxicosis of poultry: Its characteristics, prevention and reduction.
Veterinary Research Communications, 10: 429-443.
Denli, M., Okan, F. and Doran, F. 2004. Effect of conjugated linoleic acid (CLA) on the performance
and serum variables of broiler chickens intoxicated with aflatoxin B1. South African Journal of Animal Science, 34: 97- 103.
Ehrich, M., Acha, M. and Larsen, C. 1988. Interactions of aflatoxin and the antioxidant butylated hydroxytoluene in two- week- old chicks. Veterinary Research Communications, 12: 329- 333.
Ehrich, M., Driscoll, C. and Larsen, C. 1986. Ability of ethoxyquin and butylated hydroxytoluene to counteract deleterious effects of dietary aflatoxin in chicks. Avian Diseases, 30: 802-807.
Hocman, G. 1988. Chemoprevention of cancer: phenolic antioxidants (BHT, BHA). International Journal of Biochemistry, 20: 639-651.
Jindal, N., Manipal, S.K. and Mahajan, N.K. 1994. Toxicity of aflatoxin B1 in broiler chicks and its reduction by activated charcoal. Research in Veterinary Science, 56: 37-40.
Klein, P.J., Van-Vleet, T.R., Hall, J.O. and Coulombe, Jr.R.A. 2002. Dietary butylated hydroxytoluene protects against aflatoxicosis in turkeys. Toxicology and Applied Pharmacology, 182: 11-19.
Kubena, L.F., Harvey, R.B., Bailey, R.H., Buckley, S.A. and Rottinghaus, G.E. 1998. Effects of sodium calcium aluminosilicate (T-Bind) on mycotoxicosis in young broiler chickens. Poultry Science, 77: 1502-1509.
Larsen, C., Ehrich, M., Doriscoll, C. and Gross, W.B. 1985. Aflatoxin-antioxidant effects on growth of young chicks. Poultry Science, 64: 2287-2291.
Maurice, D.V., Bodine, A.B. and Rehrer, N.J. 1983. Metabolic effects of low aflatoxin B1 levels on broiler chickens. Applied and Environmental Microbiology, 45: 890.
Miazzo, R., Rosa, C.A., DeQueiroz Carcalho, E. C., Magnoli, C., Chiacchiera, S.M., Palacio, G., Saenz, M., Kikot, A., Basaldella, E. and Dalcero, A. 2000. Efficacy of synthetic zeolite to reduce the toxicity of aflatoxin in broiler chicks. Poultry Science, 79: 1-6.
Oguz, H., Kurtoglu, V. and Coskun, V. 2000. Preventive efficacy of clinoptilolite in broilers during chronic aflatoxin (50 and 100 ppb) exposure. Research in Veterinary Science, 69: 197-201.
Oguz, H., Kececi, T., Birdane, Y.O., Onder, F. and Kurtoglu, V. 2000a. Effect of clinoptilolite on serum biochemical and haematological characters of broiler chickens during experimental aflatoxicosis. Research in Veterinary Science, 69: 89-93.
Pons, W.A., Cucullu, A.F., Lee, L.S., Robertson, J.A., Franz, A.O. and Goldbatt, L.A. 1966. Determination of aflatoxins in agricultural products: Use of aqueous acetone for extraction. Journal of the AOAC, 45: 554-562.
Raju, M.V.L.N. and Devegowda, G. 2000. Influence of esterified-glucomannan on performance and organ morphology, serum biochemistry and haematology in broilers exposed to individual and combined mycotoxicosis (aflatoxin, ochratoxin and T-2 toxin). British Poultry Science, 41: 640- 50.
Rosa, C.A.R., Miazzo, R., Magnoli, C., Salvano, M., Chiacchiera, S.M., Ferrero, S., Saenz, M., Carvalho, E.C.Q. and Dalcero, A. 2001. Evaluation of the efficacy of bentonite from the south of Argentina to ameliorate the toxic effects of aflatoxin in broilers. Poultry Science, 80: 139-144.
Shashidhara, R.G. and Devegowda, G. 2003. Effect of mannanoligosaccharide on broiler breeder production traits and immunity. Poultry Science, 82: 1319- 1325.
Shi, Y.H., Xu, X.R., Feng, J.L. and Wang, C.Z. 2006. Efficacy of modified montmorillonite nanocomposite to reduce the toxicity of aflatoxin in broiler chicks. Animal Feed Science and Technology, 129: 138- 148.
Shotwell, O.L., Hesseltine, C.V., Stubblefield, R.D. and Sorenson, W.G. 1966. Production of aflatoxin on rice. Applied Microbiology, 14: 425-429.
Singletary, K.W. 1990. Effect of dietary butylated hydroxytoluene on the in vivo distribution, metabolism and DNA-binding of 7,12-dimethylbenz (a)anthracene. Cancer Letters, 49: 187-193.
Snedecor, G.W. and Cochran, W.G. 1980. Statistical Methods, 6th ed. Iowa State University Press, Ames, Iowa, USA.
Tedesco, D., Steidler, S., Galletti, S., Taneni, M., Sonzogni, O. and Ravarotto, L. 2004. Efficacy of silyrnarin-phospholipid complex in reducing the toxicity of aflatoxin B1 in broiler chicks. Poultry Science, 83: 1839- 1843.
Ulland, B.M., Weisburger, J.H., Yamamoto, R.S. and Weisburger, E.K. 1973. Antioxidants and carcinogenesis: butylated hydroxytoluene, but not diphenyl-p-phenylenediamine, inhibita cancer induction by N-2-fluorenylacetamide and by N-hydroxy-N-2-fluorenylacetamide in rats. Food and Cosmetics Toxicology, 11: 199- 207.
Williams, G.M. and Iatropoulos, M.J. 1996. Inhibition of the hapatocarcinogenicity of aflatoxin B1 in rats by low levels of the phenolic antioxidants butylated hydroxyanisole and butylated hydroxytoluene. Cancer Letters, 104: 49-53.








