Introduction
Mycotoxins are toxic metabolites that synthesized by a variety of fungal species and can be produced in feed, feedstuffs and foods in suitable conditions, such as moisture, temperature, oxygen and duration (Saleemi et al., 2017). The most common mycotoxin found in poultry feed and foodstuffs is aflatoxins (AFs) because it is produced rapidly and more toxic than the others (Oguz, 2016; Yalcin et al., 2017; Khatoon et al., 2017). AFs are a major concern in poultry production and public health because of serious economic losses and health problems. AF contamination causes reduced feed quality and reduced animal efficiency either through poor conversion of nutrients or problems such as reproductive abnormalities (Celik et al., 2000; Oguz and Kurtoglu, 2000). Aflatoxicosis in poultry also causes listlessness, anorexia with lowered growth rate; poor feed utilization, decreased egg production and increased mortality. Additionally, anemia, reduction of immune function (Oguz et al., 2003), hepatotoxicosis, hemorrhages (Ortatatli and Oguz, 2001), teratogenesis, carcinogenesis and mutagenesis are associated with aflatoxicosis (Hameed et al., 2013; Khan et al., 2013; 2017a; 2017b).
The problem of aflatoxicosis is not so easy to solve and requires constant attention throughout the entire process of grain harvest, shipping, storage, feed manufacturing, and its formulation. Nevertheless, complete avoidance of mycotoxins is not possible. Prevention of feed, feedstuffs and foods from AF contamination and utilization of AF-contaminated feed and feedstuffs presents a major problem. Detoxification, as well as routine AF analysis of feed ingredients, is an important step in a control program at field level. Detoxification is defined as neutralization, elimination or mitigation of toxic effects of mycotoxins including AFs. Still, this is quite difficult because AFs are resistant molecules. Conventionally, detoxification strategies are based on chemical, physical or microbiological methods (Devreese et al., 2013).
One of the important approaches to the prevention of mycotoxicosis in livestock is the addition of non-nutritional adsorbents in the diet that bind mycotoxins in the gastrointestinal tract, and that are capable of reducing their bioavailability. These binding agents do not undergo any changes in the digestive system, and when used to feed in different levels they prevent mycotoxins from being absorbed through the digestive system and thereby generation of toxic effects on animals and transmission of toxins into animal products. They also bind AF molecules and reduce their toxic effects (Oguz and Kurtoglu, 2000; Bhatti et al., 2017). Both inorganic and organic absorbers are used to control of mycotoxins including AF.
Inorganic mycotoxin binders include commonly aluminosilicate minerals (clays) that are the largest class of mycotoxin binders, and most of the studies on the alleviation of mycotoxicosis by the use of adsorbents have been focused on these clays (Santos et al., 2011). The organic binders or biopolymers are complex indigestible carbohydrates (cellulose, polysaccharides in the cell walls of yeast, and bacteria such as glucomannans, peptidoglycans, and others), and synthetic polymers such as cholestyramine can adsorb mycotoxins (Oguz, 2016). Saccharomyces cerevisiae initially used as a growth promoter in the early 1990's, was also found to induce beneficial effects on weight gain and immune response in broilers exposed to mycotoxins. The beneficial effects of yeast have been attributed to mannan in the yeast cell wall. By using only yeast cell walls (composed of beta-glucans and mannan oligosaccharides) instead of the whole cell, mycotoxin binding can be enhanced (Karaman et al., 2005).
The properties of adsorbents, mycotoxins and feed/food components play an important function in binding of mycotoxins and adsorbent activity. The physicochemical properties of the adsorbents such as total charge, charge distribution, size of the pores on the surface and surface area, iodine number, methylene blue index and pH as well take on an important function in binding effectively (Lemke et al., 2001). On the other hand, the properties of mycotoxins such as polarity, solubility, form and size of the mycotoxin to be adsorbed and the presence of ionized compounds in the environment are other effective factors. It has also been mentioned that the high fiber content of the feed/food substrate increased the mycotoxin affinity to adsorbent (Lemke et al., 2001). European Food Safety Authority (EFSA) stated that along with efficacy testing of mycotoxin binders; their safety should also be investigated because the toxin binders added to the feeds are thought to make non-specific bindings (EFSA, 2010).
In vitro tests provide important data for the adsorption mechanism of a substance and its validity in in vivo. Several techniques have been used to evaluate in vitro mycotoxin binding or adsorption (Ledoux and Rottinghaus, 1999). Although the in vitro adsorption tests do not give definitive results and may not always be a reliable indicator on the binding of specific mycotoxins in in vivo studies, they are used to determine a strategic mechanism for adsorbent with the identification of approximate dosage requirements for the adsorbent to be used. Therefore, in this study, it is aimed to determine the effectiveness of TBs participating in the binding of AFB1 by using organic, inorganic and mixed TBs that are widely used in the market to bind AFB1 in poultry feed.
Materials and methods
In the present work, a total of 20 toxin binders (TBs) were used and the trade name of the binders was kept confidential. TBs were divided into 3 groups according to their composition: Inorganic TBs (6), Organic TBs (6) and Mixed TBs (8). Inorganic TBs were grouped to include Ca and Al silicates, bentonite, zeolite, sepiolite, clinoptilolite etc., organic TBs were grouped to include cell wall of yeast, glucomannan, oligosaccharides, organic acid etc., and mixed TBs were grouped to include both of them (Table 1).
Table 1: Contents of toxin binders used in the study
The solutions of citrate buffer (pH 3) and phosphate buffer (pH 6.8) were used to create in vitro medium conditions compatible with gastrointestinal system of poultry. For citrate buffer (0.1 Mol/L) solution, 4.27g of trisodium citrate 2-hydrate was dissolved in 900ml of distilled water, and 17.96g of citric acid was completed to 1000ml of distilled water until the pH was 3. For the phosphate buffer, (0.1 Mol/L) 35.814g of sodium phosphoric acid was dissolved in 1000ml of distilled water and the pH was adjusted to 6.8 with phosphoric acid. The pH 3; used in the present study can be set up in the gizzard and proventriculus of poultry for a short period while pH 6.5 is found in other regions of the gastrointestinal tract and has real relevance (Leeson and Summers, 2001).
Each of the in vitro mediums produced with pH 3 and 6.8 was divided into 2 control groups and 6 experimental groups (Table 2). The same procedure was reiterated for each of 20 TBs. Analysis was performed on a total of 112 samples (8 control, 6 organic, 6 inorganic and 8 mix), repeating twice (2 for pH 3 and 2 for 6.8). The methods defined by Ledoux and Rottinghaus (2007) were used to perform in vitro analyses. For the control groups with pH 3, 0.1 Mol/L of citrate buffer solution (10ml) was put in the test tube and AFB1 dilutions were added to make 10 ppb AFB1 final concentration. For the experimental groups with the same pH, 10mg of TBs (inorganic, organic and mixed) was added into 3 tubes containing the medium prepared in the same manner. Then the samples were placed in a magnetic mixer at 41ºC for 90 minutes. At the end of 90 minutes, they were centrifuged at 1000 g at 25ºC for 5 mins., and 1ml of supernatant was collected into vials for toxin analysis, and analyzed with the HPLC-FLD device. The same procedures were repeated for the control and experimental groups prepared with 0.1 Mol/L of phosphate buffer solution adjusted to pH 6.8.
Binding percentage was determined from the amount of unbound AFB1 remaining in the supernatant (after AFB1 binding assay) compared to control (AFB1 without TB):
Ab = 100 [1- A1/A0]
Ab = % of AFB1 bound
A1= Amount of AFB1 in supernatant after binding assay
A0 = Amount of AFB1 in control
The toxin was assessed by Vicam Aflatest AFB1 analysis method. HPLC-FLD device (Agilent 1260 Series, Boblingen, Germany; Column ACE 5 C18) conditions: Mobile phase; Water: Methanol: Acetonitrile (58: 27, 8: 14, 2; v/v/v); flow rate: 1 ml/min.; pressure: 140-150 bar; column temperature: 30ºC; analysis time: 16 mins.; injection volume: 100μL; retention time for AFB1: 14.4 mins; derivatization unit: Photochem. Validation parameters for the method: LOD 0.16 ppb; LOQ 0.54 ppb; recovery (R) 90%, measurement uncertainty±0.25 (Magnusson and Ornemark, 2014).
Study data was presented mean±SE and evaluated by ANOVA and Tukey test as a posthoc (SPSS, 22.0). P<0.05 level was accepted statistical significance.
Table 2: Study design in in vitro condition
Results
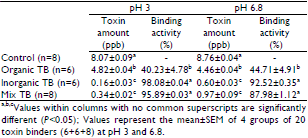
At the end of the analysis on 3 groups of TBs in in vitro gastrointestinal mediums of poultry, the most effective TB was inorganic TB based on the percentage of binding AFB1 at the 10 ppb level. The effectivity of inorganic and mix TBs was around 95-100% and organic was 40-45% to bind AFB1.
Inorganic TBs had the highest capacity to bind AFB1 with 98% and the organic TBs had the lowest with 40% used four poultry medium under in vitro at pH 3 conditions (Table 3). Similarly, inorganic TBs had the highest capacity to bind AFB1 with 93% and the organic TBs had the lowest with 45% under in vitro at pH 6.8 conditions. In the study, increased pH of in vitro conditions for inorganic TBs numerically reduced the capacity of binding (P>0.05) (98 versus 93%), whereas there was an increase in the same rate in organic TBs (P>0.05) (40 versus 45%). The capability of mixed TBs to bind AFB1 was found 96% at pH 3, and reduced 88% when pH increased to 6.8 (P>0.05). According to the result the inorganic and mixed TBs were more effective than organic TBs to bind AFB1 (Table 3).
The difference between three types of TBs (inorganic, organic and mix) was significant statistically in terms of binding activity to AFB1 (P<0.05). Besides, the difference between organic, inorganic and mixed TB was found to be significant at both pH 3 and pH 6.8 (P<0.05). It was found no statistical differences within the groups and differences between two pH values were not statistically significant (P>0.05). Accordingly, it was found no significant differences within TBs themselves and at both pH 3 and pH 6.8 (P>0.05; Table 3).
Table 3: Unbond AFB1 levels and the binding effect of toxin binders (Mean±SEM).
Discussion
In the present study, it was investigated whether organic, inorganic and mix TBs were effective in binding of AFB1 in in vitro conditions. Inorganic and mix TBs were found to be effective on AFB1 binding among the TBs used (Table 3). These results are consistent with several pieces of research.
Inorganic TBs used in this study mainly contain Ca and Al silicates, zeolite, bentonite, clinoptilolite and sepiolite. Inorganic TBs were also reported to induce a higher binding effect on AFB1 in the other research. Similarly, other inorganic TBs used as a feed additive such as HSCAS (Neeff et al., 2013), montmorillonite (Shi et al., 2009) and clinoptilolite (Oguz and Kurtoglu, 2000; Ortatatli and Oguz, 2001) found to be effective to adsorb AFB1 both in vitro and in vivo conditions. Diaz et al. (2002) demonstrated that the binding ability of three different Na bentonite to AFB1 (5 μg/ml) was between 95.1% and 98.4%, and Ca bentonite had 98.5% AFB1 binding ability in a study conducted in vitro. However, Vekiru et al. (2015) reported that zeolites used in in vitro condition were ineffective in AFB1 binding, while Ca and Na bentonites were effective.
The properties of adsorbents, mycotoxins and feed/food ingredients play an important function in binding activity of adsorbents. Generally, the binding capacity increases with surface area and chemical affinities between adsorbent and mycotoxin (Devreesse et al., 2013). The activity of inorganic TBs in AFB1 binding may be due to the fact that the clays are "compressible" between the silicate layers and that the cations bind interchangeable with the hydrogen bond (Deng et al., 2010). Moreover, those different inorganic TBs bind AFB1 more effectively than others (EFSA, 2011) may be the consequence of the purification of inorganic clays which are in different chemical composition (Diaz et al., 2002). While mycotoxins with a polar functional group such as AF are effectively adsorbing by the adsorbents such as montmorillonite and zeolite, relatively apolar mycotoxins such as zearalenone and ochratoxin A cannot bind strongly by (-) charged adsorbents.
Organic TBs were found to be the less effective on AFB1 binding among the used TBs in this study (Table 3). Organic TBs used in this study, mainly contain live yeast, glucomannan/esterified glucomannan and mannanoligo-saccharide. There are also some reports that organic TBs such as live yeast (Saccharomyces cerevisiae), gluco-mannan, mannanoligo-saccharide are effective in binding of AFB1 in feed at feeding trials performed in vivo. It was mentioned that the adverse effects of AFB1 were reduced by the addition of feed glucomannan (Karaman et al., 2005; Uttpatel et al., 2011) and mannanoligo-saccharide (Attia et al., 2013) in in vivo feeding trials. Live yeast strains were found to be efficient in binding of AF after mixing as a feed additive (Dogi et al., 2011). Likewise, in vitro studies have demonstrated that yeast cells bind AFB1 up to 90%, depending on the level. However, in the present study, it was noticed that the binding capacity of organic TBs was low in in vitro conditions. The difference between our results and other studies might have been resulted from the types, contents and levels of organic TBs, AF concentration and/or other conditions available.
Mix TBs were also found to be effective on AFB1 binding among the used TBs in this study. They mainly contain aluminosilicates, zeolite, bentonite, sepiolite, clinoptilolite, yeast wall components, glucomannan, mannaoligo-saccharide, organic acid, plant extracts and their mixture. The binding efficacy of mixed TBs in this study may be due to inorganic toxin binders which are present in their compositions. Adsorption efficiency of mixed adsorbent is based on the creation of a hydrated bond between AF and the inorganic component of the adsorbent. In the present study, the effect of pH on the binding activity of TBs for AFB1 was also assessed. It was observed that binding activity at pH 3 was numerically higher, except organic TBs (Table 3). In the control group, it was observed that 19.3% and 12.24% of AFB1 were degraded at pH 3 and pH 6.8 in in vitro condition, respectively. These losses may have been due to the medium used and the procedures being performed.
The primary advantage of conducting an in vitro test is to be able to demonstrate if a sequestering agent has little or no affinity for AFB1. If the agent does not bind AFB1 in vitro, it is unlikely to bind in in vivo environment. As in vitro preliminary tests of mycotoxin adsorption are regarded as a potent tool for screening potential mycotoxin-detoxifying agents since if no adsorption occurs in vitro, little or no chance exists to do so in vivo (Pappas et al., 2014). Ledoux and Rottinghaus (1999) stated that mycotoxin adsorbents with a binding ability higher than 80%, under in vitro conditions, may be considered for in vivo evaluation for binding of mycotoxin in the feed. According to this, inorganic and mix TBs in this study, may be used as a feed additive to bind AF in in vivo.
Since the early 1990s, experiments with adsorbents such zeolites and aluminosilicates have proven successful as a feed additive, but high inclusion rates and possible potential interactions with feed nutrients are causes for concern (Oguz and Kurtoglu, 2000; EFSA, 2010). Therefore, The European Commission launched a new group of technological feed additives for the reduction of mycotoxins in feed to overcome this unsatisfactory legal situation. In 2010, EFSA published guidelines with stringent requirements (EFSA, 2010; Murugesan et al., 2015). Toxin binders used in the feed should be inert and non-toxic and have no pharmacological and toxicological effects themselves into animals. Possible nutrient interaction and dioxin and heavy metal contaminations should also be regarded for using of natural clays (Oguz, 2016).
This study showed that inorganic TBs and also the mix TBs were effective in binding of AFB1 in in vitro poultry gastrointestinal conditions. These results may lead to further in vivo studies. This research may also provide a more conscious approach to control AFB1 in feed and TBs to be used in feed and livestock industry.
Acknowledgments: This study was funded by Ministry of Food, Agriculture and Livestock; Project no: TAGEM-HSGYAD 14/AO5/PO4/69. This paper was presented as a poster in 32nd World Veterinary Congress, 13-17 September 2015, Istanbul, Turkey.
Authors contribution: NFY: Design of research, determination and collection of toxin binders, research process and toxicological analyzes carried out in the laboratory, writing of the paper. TA: Design of research, determination and collection of toxin binders, research process and toxicological analyzes carried out in the laboratory. MKI: Design of research, determination and collection of toxin binders, research process and toxicological analyzes carried out in the laboratory. HO: Design of research, following of research process and toxicological analyzes carried out in the laboratory, writing and editing of the paper.
This article was originally published in Pakistan Veterinary Journal, 38(1): 61-65. http://dx.doi.org/10.229261/pakvetj/2018.012.