Dietary spray-dried plasma improves intestinal morphology of mated female mice under stress condition
Author details:
Background: Stress causes inflammation that impairs intestinal barrier function. Dietary spray-dried plasma (SDP) has recognized anti-inflammatory effects and improvement of gut barrier function. Therefore, the purpose of this study was to investigate the effects of dietary SDP on intestinal morphology of mated female mice under stress condition.
Results: Villus height, width, and area of small intestines were low on gestation day (GD) 3 or 4 under stress conditions, and higher later (Time, P < 0.05). Crypt depth of colon was low on GD 4 and higher later (Time, P < 0.05). Meanwhile, the SDP treatments improved (P < 0.05) intestinal morphology, indicated by increased villus height, villus width, villus area, and ratio between villus height and crypt depth of small intestines and crypt depth of colon, and by decreased crypt depth of small intestines, compared with the control diet. The SDP treatments also increased (P < 0.05) the number of goblet cells in intestines compared with the control diet. There were no differences between different levels of SDP.
Conclusion: Dietary SDP improves intestinal morphology of mated female mice under stress condition.
Keywords: Intestinal morphology, Mated female mice, Spray-dried plasma, Stress.
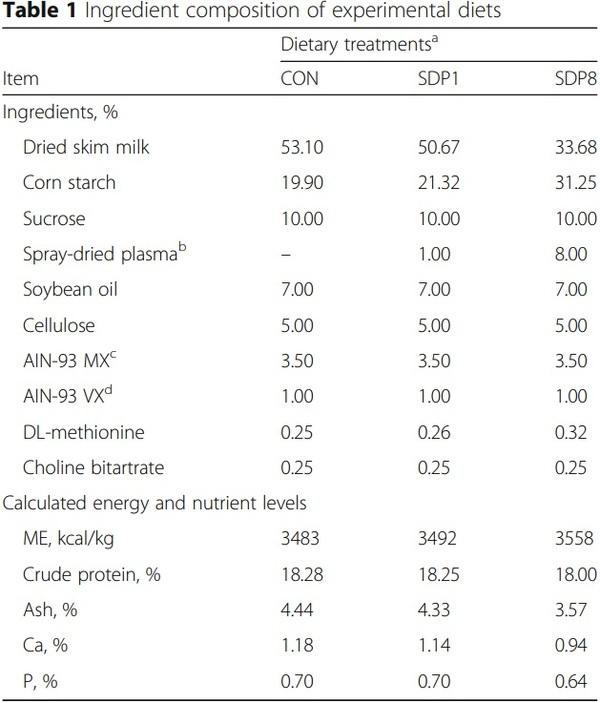
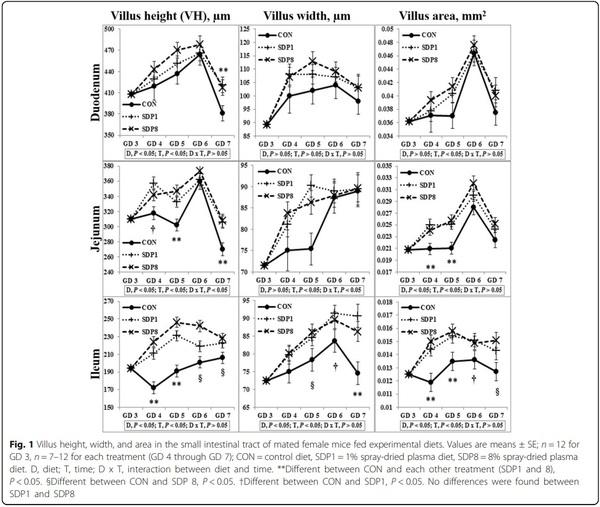
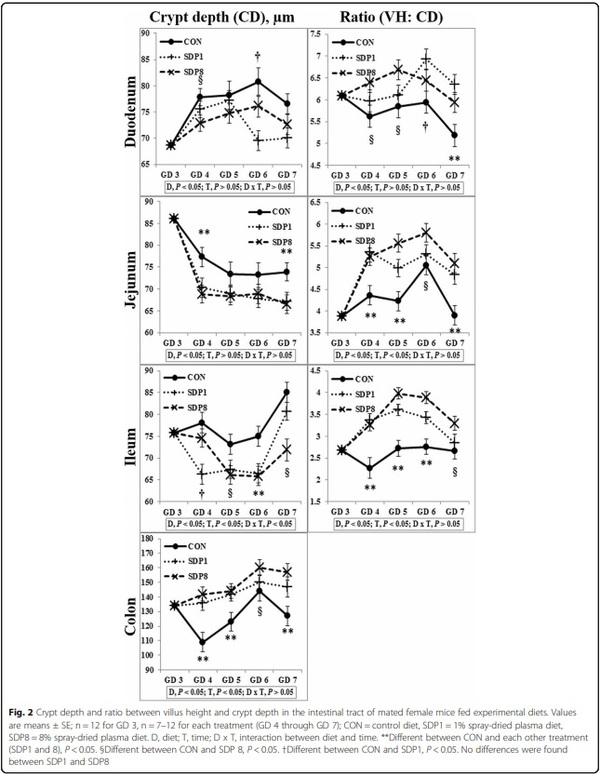
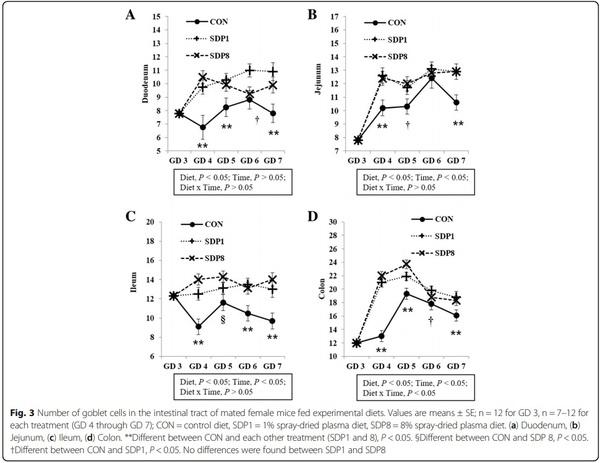
1. Coffey RD, Cromwell GL. Use of spray-dried animal plasma in diets for weanling pigs. Pig News Info. 2001;22:39–48.
2. Song M, Liu Y, Lee JJ, Che TM, Soares-Almeida JA, Chun JL, Cambell JM,
Polo J, Crenshaw JD, Seo SW, Pettigrew JE. Spray-dried plasma attenuates
Fig. 3 Number of goblet cells in the intestinal tract of mated female mice fed experimental diets. Values are means ± SE; n = 12 for GD 3, n = 7–12 for each treatment (GD 4 through GD 7); CON = control diet, SDP1 = 1% spray-dried plasma diet, SDP8 = 8% spray-dried plasma diet. (a) Duodenum, (b)
Jejunum, (c) Ileum, (d) Colon. **Different between CON and each other treatment (SDP1 and 8), P < 0.05. §Different between CON and SDP 8, P < 0.05.
†Different between CON and SDP1, P < 0.05. No differences were found between SDP1 and SDP8
Liu et al. Journal of Animal Science and Technology (2018) 60:10 Page 5 of 6 inflammation and improves pregnancy rate of mated female mice. J Anim
Sci. 2015;93:298–305.
3. Jang K, Kim J, Kim S, Jang Y, Lee J, Kim Y, Park J, Kim Y, Song M. Value of spray-dried plasma as a supplement to swine diets. Korean J Agric Sci. 2016;
43:14–20.
4. Pettigrew JE. Reduced use of antibiotic growth promoters in diets fed to weanling pigs: dietary tools, part 1. Anim Biotechnol. 2006;17:207–15.
5. Nollet H, Deprez P, Van Driessche E, Muylle E. Protection of just weaned pigs against infection with F18+ Escherichia coli by non-immune plasma powder. Vet Microbiol. 1999;65:37–45.
6. Niewold TA. The nonantibiotic anti-inflammatory effect of antimicrobial growth promoters, the real mode of action? A Hypothesis Poul Sci. 2007;86:
605–9.
7. Pérez-Bosque A, Pelegrí C, Vicario M, Castell M, Russell LE, Campbell JM,
Quigley JD, Polo J, Amat C, Moretό M. Dietary plasma protein affects the immune response of weaned rats challenged with S. aureus superantigen B.
J Nutr. 2004;134:2667–72.
8. Peace RM, Campbell JM, Polo J, Crenshaw JD, Russell LE, Moeser RL. Spraydried porcine plasma influences intestinal barrier function, inflammation, and diarrhea in weaned pigs. J Nutr. 2011;141:1312–7.
9. Pérez-Bosque A, Amat C, Polo J, Campbell JM, Crenshaw JD, Russell LE,
Moretό M. Spray-dried animal plasma prevents the effects of Staphylococcus aureus enterotoxin B on intestinal barrier function in weaned rats. J Nutr.
2006;136:2838–43.
10. Crenshaw JD, Boyd RD, Campbell JM, Russell LE, Moeser RL, Wilson ME.
Lactation feed disappearance and weaning to estrus interval for sows fed spray-dried plasma. J Anim Sci. 2007;85:3442–53.
11. Frugé ED, Roux ML, Lirette RD, Bidner TD, Southern LL, Crenshaw JD. Effects of dietary spray-dried plasma protein on sow productivity during lactation. J
Anim Sci. 2009;87:960–4.
12. Pérez-Bosque A, Mirό L, Polo J, Russell LE, Campbell JM, Weaver E,
Crenshaw JD, Moretό M. Dietary plasma proteins modulate the immune response of diffuse gut-associated lymphoid tissue in rats challenged with
Staphylococcus aureus enterotoxin B. J Nutr. 2008;138:533–5337.
13. Pérez-Bosque A, Mirό L, Polo J, Russell LE, Campbell JM, Weaver E,
Crenshaw JD, Moretό M. Dietary plasma protein supplements prevent the release of mucosal proinflammatory mediators in intestinal inflammation in rats. J Nutr. 2010;140:25–30.
14. Cohen S, Miller GE, Rabin BS. Psychological stress and antibody response to immunization: a critical review of the human literature. Psychosom Med.
2001;63:7–18.
15. Lambert GP. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J Anim Sci. 2009;87:101–8.
16. Moretó M, Pérez-Bosque A. Dietary plasma proteins, the intestinal immune system, and the barrier functions of the intestinal mucosa. J Anim Sci. 2009;
87:92–100.
17. Yu J, Yin P, Liu F, Cheng G, Guo K, Lu A, Zhu X, Luan W, Xu J. Effect of heat stress on the porcine small intestine: a morphological and gene expression study. Comp Biochem Physiol. 2010;156:119–28.
18. Liptrap RM. Stress and reproduction in domestic animals. Ann N Y Acad Sci.
1993;697:275–84.
19. Einarsson S, Brandt Y, Lundeheim N, Madej A. Stress and its influence on reproduction in pigs: a review. Acta Vet Scand. 2008;50(48):1–3.
20. Owusu-Asiedu A, Nyachoti CM, Baidoo SK, Marquardt RR, Yang X. Response of early-weaned pigs to an enterotoxigenic Escherichia coli (K88) challenge when fed diets containing spray-dried porcine plasma or pea protein isolate plus egg yolk antibody. J Anim Sci. 2003;81:1781–9.
21. Owusu-Asiedu A, Nyachoti CM, Marquardt RR. Response of early-weaned pigs to an enterotoxigenic Escherichia coli (K88) challenge when fed diets containing spray-dried porcine plasma or pea protein isolate plus egg yolk antibody, zinc oxide, fumaric acid, or antibiotic. J Anim Sci. 2003;81:1790–8.
22. Yi GF, Carroll JA, Allee GL, Gaines AM, Kendall DC, Usry JL, Toride Y, Izuru S.
Effect of glutamine and spray-dried plasma on growth performance, small intestinal morphology, and immune responses of Escherichia coli K88+ - challenged weaned pigs. J Anim Sci. 2005;83:634–43.
23. NRC. Nutrient requirements of laboratory animals. 4th ed. Washington: The
National Academics Press; 1995.
24. Balan P, Han KS, Rutherfurd SM, Singh H, Moughan PJ. Orally administered ovine serum immunoglobulins influence growth performance, organ weights, and gut morphology in growing rats. J Nutr. 2008;139:244–9.
25. Yamauchi K, Yamamoto K, Ishiki Y. Morphological alterations of the intestinal villi and absorptive epithelial cells in each intestinal part in fasted chickens. Jpn Poult Sci. 1995;32:241–51.
26. Yamauchi K, Kamisoyama H, Ishiki Y. Effects of fasting and refeeding on structures of the intestinal villi and epithelial cells in white leghorn hens. Br
Poult Sci. 1996;37:909–21.
27. Burkholder KM, Thompson KL, Einstein ME, Applegate TJ, Patterson JA.
Influence of stressors on normal intestinal microbiota, intestinal morphology, and susceptibility to Salmonella Enteritidis colonization in broilers. Poult Sci. 2008;87:1734–41.
28. Santos J, Saunders PR, Hanssen NP, Yang PC, Yates D, Groot JA, Perdue MH.
Corticotropin-releasing hormone mimics stress-induced colonic epithelial pathophysiology in the rat. Am J Phys. 1999;277:391–9.
29. Saunders PR, Kosecka U, McKay DM, Perdue MH. Acute stressors stimulate ion secretion and increase epithelial permeability in rat intestine. Am J Phys.
1994;267:794–9.
30. Nofrarías M, Manzanilla EG, Pujols J, Gibert X, Majó N, Segalés J, Gasa J.
Spray-dried porcine plasma affects intestinal morphology and immune cell subsets of weaned pigs. J Anim Sci. 2006;84:2735–42.














