In Vitro Rumen Simulations Show a Reduced Disappearance of Deoxynivalenol, Nivalenol and Enniatin B at Conditions of Rumen Acidosis and Lower Microbial Activity
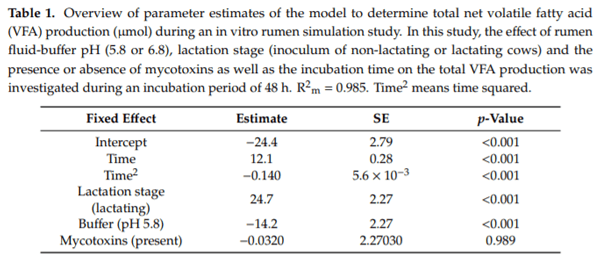
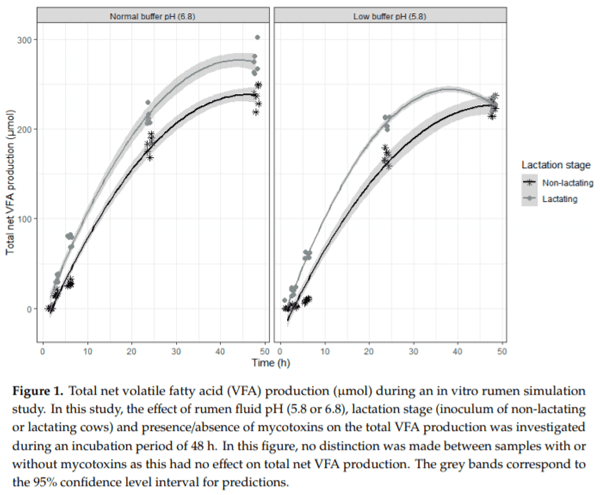
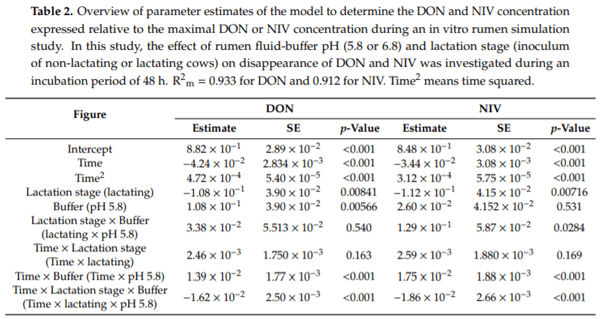
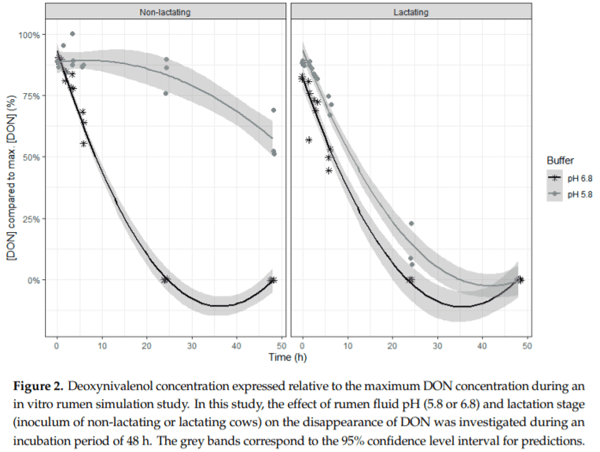
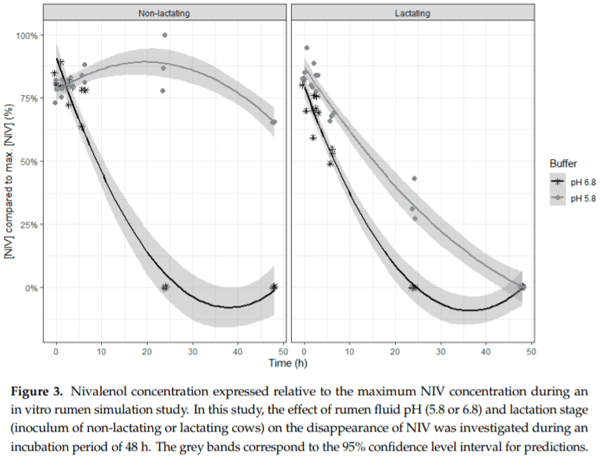
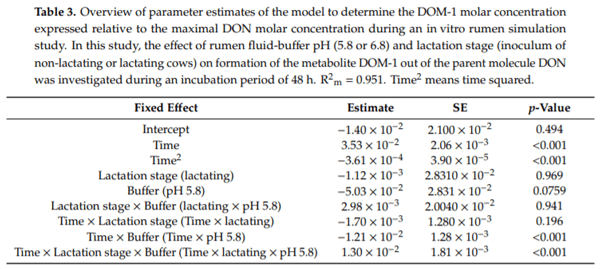
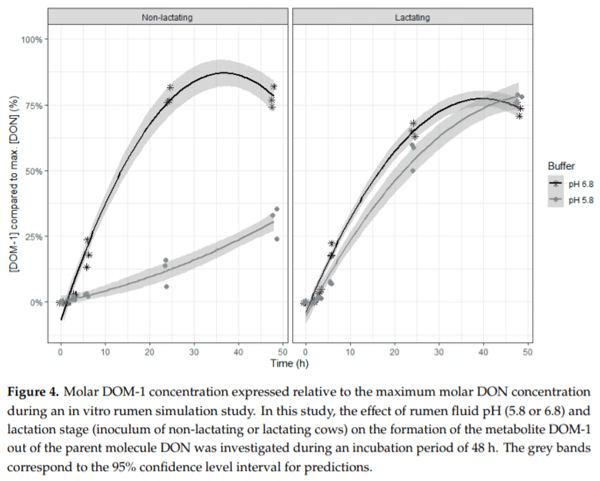
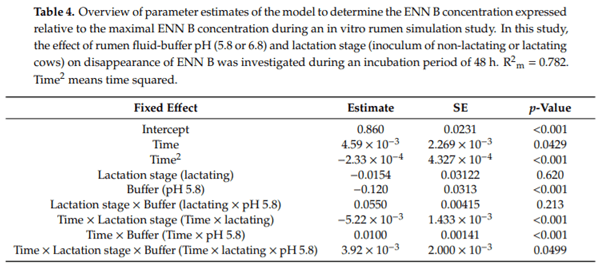
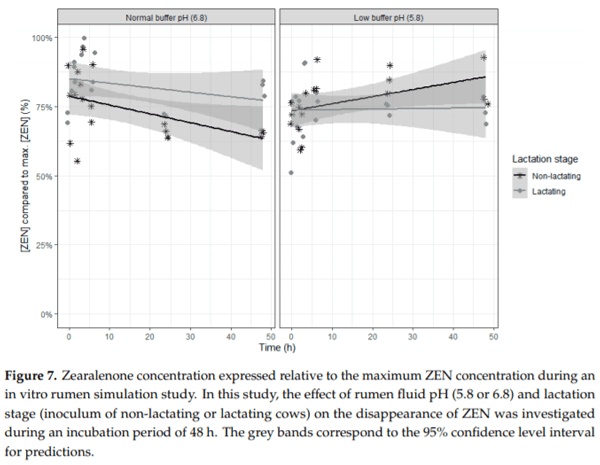
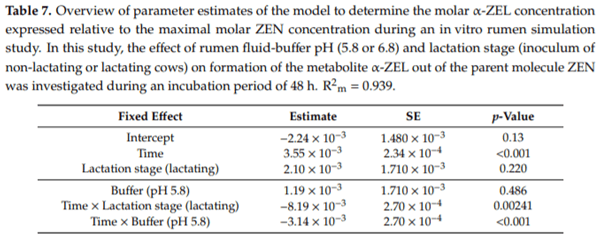
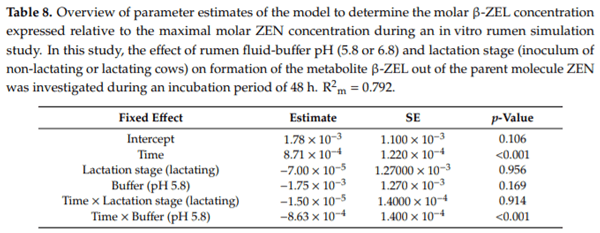
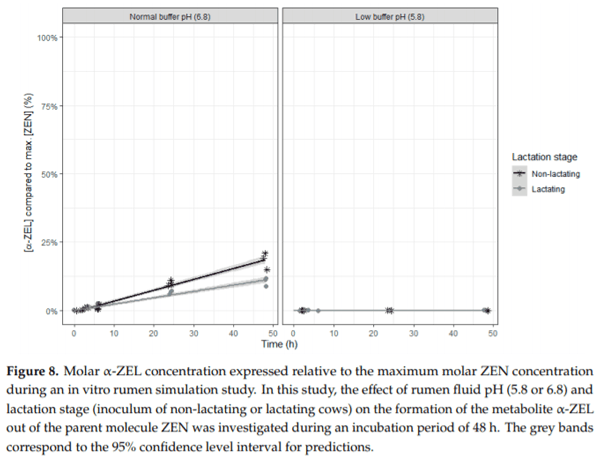
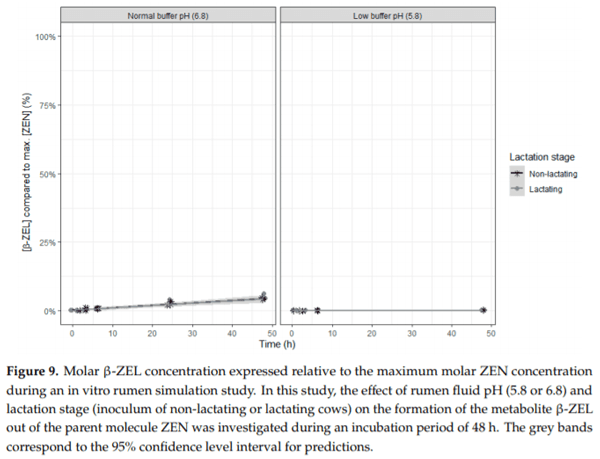
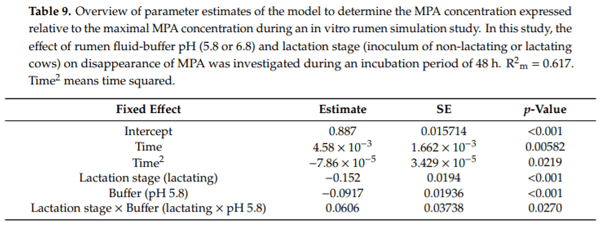
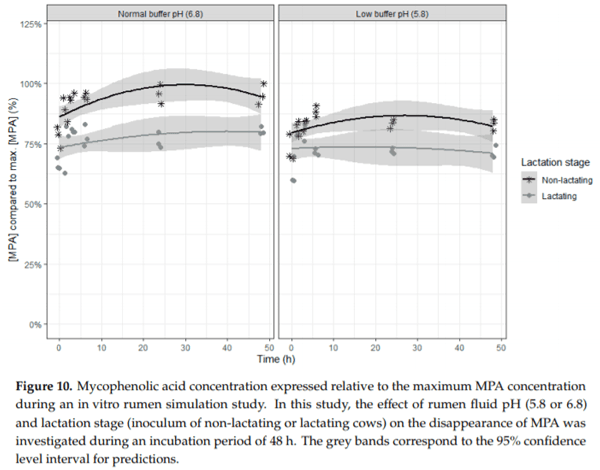
Ruminants are generally considered to be less susceptible to the effects of mycotoxins than monogastric animals as the rumen microbiota are capable of detoxifying some of these toxins. Despite this potential degradation, mycotoxin-associated subclinical health problems are seen in dairy cows. In this research, the disappearance of several mycotoxins was determined in an in vitro rumen model and the effect of realistic concentrations of those mycotoxins on fermentation was assessed by volatile fatty acid production. In addition, two hypotheses were tested: (1) a lower rumen pH leads to a decreased degradation of mycotoxins and (2) rumen fluid of lactating cows degrade mycotoxins better than rumen fluid of non-lactating cows. Maize silage was spiked with a mixture of deoxynivalenol (DON), nivalenol (NIV), enniatin B (ENN B), mycophenolic acid (MPA), roquefortine C (ROQ-C) and zearalenone (ZEN). Fresh rumen fluid of two lactating cows (L) and two non-lactating cows (N) was added to a buffer of normal pH (6.8) and low pH (5.8), leading to four combinations (L6.8, L5.8, N6.8, N5.8), which were added to the spiked maize substrate. In this study, mycotoxins had no effect on volatile fatty acid production. However, not all mycotoxins fully disappeared during incubation. ENN B and ROQ-C disappeared only partially, whereas MPA showed almost no disappearance. The disappearance of DON, NIV, and ENN B was hampered when pH was low, especially when the inoculum of non-lactating cows was used. For ZEN, a limited transformation of ZEN to α-ZEL and β-ZEL was observed, but only at pH 6.8. In conclusion, based on the type of mycotoxin and the ruminal conditions, mycotoxins can stay intact in the rumen.
Keywords: mycotoxins; maize silage; SARA; lactation stage; rumen fluid; UPLC-MS/MS
Key Contribution: This in vitro rumen simulation study provides first insights into the effect of rumen pH and inoculum on the degradation of mycotoxins by the rumen microbiota. The results emphasize the potential toxicological importance of mycotoxins in ruminants as not all mycotoxins are degraded by the rumen microbiota and degradation is also influenced by ruminal pH and microbial inoculum.
1. Yiannikouris, A.; Jouany, J.-P. Mycotoxins in feeds and their fate in animals: A review. Anim. Res. 2002, 51, 81–99. [CrossRef]
2. Fink-Gremmels, J. The role of mycotoxins in the health and performance of dairy cows. Vet. J. 2008, 176, 84–92. [CrossRef]
3. Fink-Gremmels, J. Mycotoxins in cattle feeds and carry-over to dairy milk: A review. Food Addit. Contam. Part A 2008, 25, 172–180. [CrossRef]
4. Upadhaya, S.D.; Park, M.A.; Ha, J.K. Mycotoxins and their biotransformation in the rumen: A review. Asian-Australas. J. Anim. Sci. 2010, 23, 1250–1260. [CrossRef]
5. Gallo, A.; Masoero, F. In vitro models to evaluate the capacity of different sequestering agents to adsorb aflatoxins. Ital. J. Anim. Sci. 2010, 9, 109–116. [CrossRef]
6. Peltonen, K.D.; El-Nezami, H.S.; Salminen, S.J.; Ahokas, J.T. Binding of aflatoxin B1 by probiotic bacteria. J. Sci. Food Agric. 2000, 80, 1942–1945. [CrossRef]
7. Oatley, J.T.; Rarick, M.D.; Ji, G.E.; Linz, J.E. Binding of aflatoxin B1 to Bifidobacteria in vitro. J. Food Prot. 2000, 63, 1133–1136. [CrossRef]
8. Karazhyan, R.; Shaker Sheyda, I.; Mehraban SangAtash, M.; Tajalli, F.; Mojtahedi, M.; Sadegh, M. Effect of Saccharomyces cerevisiae yeast on ruminal detoxification of aflatoxin B1. J. Vet. Res. 2017, 72, 81–86.
9. El-Nezami, H.; Kankaanpää, P.; Salminen, S.; Ahokas, J. Physicochemical alterations enhance the ability of dairy strains of lactic acid bacteria to remove aflatoxin from contaminated media. J. Food Prot. 1998, 61, 466–468. [CrossRef]
10. El-Nezami, H.; Polychronaki, N.; Salminen, S.; Mykkänen, H. Binding rather than metabolism may explain the interaction of two food-grade Lactobacillus strains with zearalenone and its derivative α-zearalenol. Appl. Environ. Microbiol. 2002, 68, 3545–3549. [CrossRef]
11. El-Nezami, H.; Polychronaki, N.; Lee, Y.K.; Haskard, C.; Juvonen, R.; Salminen, S.; Mykkänen, H. Chemical moieties and interactions involved in the binding of zearalenone to the surface of Lactobacillus rhamnosus strains GG. J. Agric. Food Chem. 2004, 52, 4577–4581. [CrossRef]
12. Driehuis, F.; Spanjer, M.C.; Scholten, J.M.; Te Giffel, M.C. Occurrence of mycotoxins in maize, grass and wheat silage for dairy cattle in the Netherlands. Food Addit. Contam. Part B 2008, 1, 41–50. [CrossRef]
13. Zachariasova, M.; Dzuman, Z.; Veprikova, Z.; Hajkova, K.; Jiru, M.; Vaclavikova, M.; Zachariasova, A.; Pospichalova, M.; Florian, M.; Hajslova, J.; et al. Occurrence of multiple mycotoxins in European feedingstuffs, assessment of dietary intake by farm animals. Anim. Feed Sci. Technol. 2014, 193, 124–140. [CrossRef]
14. Valgaeren, B.; Théron, L.; Croubels, S.; Devreese, M.; De Baere, S.; Van Pamel, E.; Daeseleire, E.; De Boevre, M.; De Saeger, S.; Vidal, A.; et al. The role of roughage provision on the absorption and disposition of the mycotoxin deoxynivalenol and its acetylated derivatives in calves: From field observations to toxicokinetics. Arch. Toxicol. 2018, 93, 293–310. [CrossRef]
15. Goff, J.P. The monitoring, prevention, and treatment of milk fever and subclinical hypocalcemia in dairy cows. Vet. J. 2008, 176, 50–57. [CrossRef]
16. Husband, J. Strategies for the control of milk fever. In Pract. 2005, 27, 88–92. [CrossRef]
17. Clauss, M.; Jürgen Streich, W.; Schwarm, A.; Ortmann, S.; Hummel, J. The relationship of food intake and ingesta passage predicts feeding ecology in two different megaherbivore groups. Oikos 2007, 116, 209–216. [CrossRef]
18. Khafipour, E.; Li, S.; Plaizier, J.C.; Krause, D.O. Rumen microbiome composition determined using two nutritional models of subacute ruminal acidosis. Appl. Environ. Microbiol. 2009, 75, 7115–7124. [CrossRef]
19. Vandicke, J.; De Visschere, K.; Croubels, S.; De Saeger, S.; Audenaert, K.; Haesaert, G. Mycotoxins in Flanders’ fields: occurrence and correlations with Fusarium species in whole-plant harvested maize. Microorganisms 2019, 7, 571. [CrossRef]
20. European Commission. Commission Recommendation No 2006/576/EC of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2006, L229, 7–9. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/ ?uri=CELEX%3A32006H0576 (accessed on 1 January 2020).
21. Tangni, E.K.; Pussemier, L.; Bastiaanse, H.; Haesaert, G.; Foucart, G.; Van Hove, F. Presence of mycophenolic acid, roquefortine C, citrinin and ochratoxin A in maize and grass silages supplied to dairy cattle in Belgium. J. Anim. Sci. Adv. 2013, 3, 598–612.
22. Driehuis, F.; Spanjer, M.C.; Scholten, J.M.; te Giffel, M.C. Occurrence of mycotoxins in feedstuffs of dairy cows and estimation of total dietary intakes. J. Dairy Sci. 2008, 91, 4261–4271. [CrossRef]
23. Wambacq, E.; Vanhoutte, I.; Audenaert, K.; De Gelder, L.; Haesaert, G. Occurrence, prevention and remediation of toxigenic fungi and mycotoxins in silage: A review. J. Sci. Food Agric. 2016, 96, 2284–2302. [CrossRef]
24. Seeling, K.; Boguhn, J.; Strobel, E.; Dänicke, S.; Valenta, H.; Ueberschär, K.H.; Rodehutscord, M. On the effects of Fusarium toxin contaminated wheat and wheat chaff on nutrient utilisation and turnover of deoxynivalenol and zearalenone in vitro (Rusitec). Toxicol. Vitr. 2006, 20, 703–711. [CrossRef]
25. Hedman, R.; Pettersson, H. Transformation of nivalenol by gastrointestinal microbes. Arch. Anim. Nutr. 1997, 50, 321–329. [CrossRef]
26. Seeling, K.; Dänicke, S.; Valenta, H.; Van Egmond, H.; Schothorst, R.; Jekel, A.; Lebzien, P.; Schollenberger, M.; Razzazi-Fazeli, E.; Flachowsky, G.; et al. Effects of Fusarium toxin-contaminated wheat and feed intake level on the biotransformation and carry-over of deoxynivalenol in dairy cows. Food Addit. Contam. 2006, 23, 1008–1020. [CrossRef]
27. Kiessling, K.H.; Pettersson, H.; Sandholm, K.; Olsen, M. Metabolism of aflatoxin, ochratoxin, zearalenone, and three trichothecenes by intact rumen fluid, rumen protozoa, and rumen bacteria. Appl. Environ. Microbiol. 1984, 47, 1070–1073. [CrossRef]
28. Dänicke, S.; Matthäus, K.; Lebzien, P.; Valenta, H.; Stemme, K.; Ueberschär, K.-H.; Razzazi-Fazeli, E.; Böhm, J.; Flachowsky, G. Effects of Fusarium toxin-contaminated wheat grain on nutrient turnover, microbial protein synthesis and metabolism of deoxynivalenol and zearalenone in the rumen of dairy cows. J. Anim. Physiol. Anim. Nutr. 2005, 89, 303–315. [CrossRef]
29. Seeling, K.; Dänicke, S.; Ueberschär, H.; Lebzien, P.; Flachowsky, G. On the effects of Fusarium toxin-contaminated wheat and the feed intake level on the metabolism and carry over of zearalenone in dairy cows. Food Addit. Contam. 2005, 22, 847–855. [CrossRef]
30. Gallo, A.; Giuberti, G.; Bertuzzi, T.; Moschini, M.; Masoero, F. Study of the effects of PR toxin, mycophenolic acid and roquefortine C on in vitro gas production parameters and their stability in the rumen environment. J. Agric. Sci. 2015, 153, 163–176. [CrossRef]
31. He, P.; Young, L.G.; Forsberg, C. Microbial transformation of deoxynivalenol (vomitoxin). Appl. Environ. Microbiol. 1992, 58, 3857–3863. [CrossRef]
32. Jeong, J.S.; Lee, J.H.; Simizu, Y.; Tazaki, H.; Itabashi, H.; Kimura, N. Effects of the Fusarium mycotoxin deoxynivalenol on in vitro rumen fermentation. Anim. Feed Sci. Technol. 2010, 162, 144–148. [CrossRef]
33. Kennedy, D.G.; Hewitt, S.A.; McEvoy, J.D.G.; Currie, J.W.; Cannavan, A.; Blanchflower, W.J.; Elliot, C.T. Zeranol is formed from Fusarium spp. toxins in cattle in vivo. Food Addit. Contam. 1998, 15, 393–400. [CrossRef] [PubMed]
34. Pell, A.N.; Schofield, P. Computerized monitoring of gas production to measure forage digestion in vitro. J. Dairy Sci. 1993, 76, 1063–1073. [CrossRef]
35. Swanson, S.P.; Nicoletti, J.; Rood, H.D., Jr.; Buck,W.B.; Cote, L.M.; Yoshizawa, T. Metabolism of three trichothecene mycotoxins, T-2 toxin, diacetoxyscirpenol and deoxynivalenol, by bovine rumen microorganisms. J. Chromatogr. 1987, 414, 335–342. [CrossRef]
36. Fuchs, E.; Binder, E.M.; Heidler, D.; Krska, R. Structural characterization of metabolites after the microbial degradation of type A trichothecenes by the bacterial strain BBSH 797. Food Addit. Contam. 2002, 19, 379–386. [CrossRef]
37. Russell, J.B.; Wilson, D.B. Why Are Ruminal Cellulolytic Bacteria Unable to Digest Cellulose at Low pH? J. Dairy Sci. 1996, 79, 1503–1509. [CrossRef]
38. Erfle, J.D.; Boila, R.J.; Teather, R.M.; Mahadevan, S.; Sauer, F.D. Effect of pH on Fermentation Characteristics and Protein Degradation by Rumen Microorganisms In Vitro. J. Dairy Sci. 1982, 65, 1457–1464. [CrossRef]
39. Slyter, L.L.; Bryant, M.P.; Wolin, M.J. Effect of pH on population and fermentation in a continuously cultured rumen ecosystem. Appl. Microbiol. 1966, 14, 573–578. [CrossRef]
40. Razzazi, E.; Böhm, J.; Ahmed, K.; Cecon, B.; Rabus, B. Investigation on the biodegradability of mycotoxins nivalenol (NIV) and deoxynivalenol (DON) in a RUSITEC fermentor and their monitoring by HPLC/MS. Mycotoxin Res. 2000, 16, 9–14. [CrossRef]
41. Zhang, Y.G.; Liu, S.; Zhao, X.J.; Wang, N.; Jiang, X.; Xin, H.S.; Zhang, Y.G. Lactobacillus rhamnosus GG modulates gastrointestinal absorption, excretion patterns, and toxicity in Holstein calves fed a single dose of aflatoxin B1. J. Dairy Sci. 2018, 102, 1330–1340. [CrossRef]
42. Debevere, S.; De Baere, S.; Haesaert, G.; Rychlik, M.; Fievez, V.; Croubels, S. In vitro rumen simulation shows detoxification of the mycotoxin deoxynivalenol and activation of the mycotoxin zearalenone, but also adhesion of mycotoxins to maize silage. In Proceedings of the 43rd Animal Nutrition Research Forum, Wageningen, The Netherlands, 11 April 2018; pp. 41–42.
43. King, R.R.; McQueen, R.E.; Levesque, D.; Greenhalgh, R. Transformation of deoxynivalenol (vomitoxin) by rumen microorganisms. J. Agric. Food Chem. 1984, 32, 1181–1183. [CrossRef]
44. Binder, E.M.; Binder, J.; Ellend, N.; Schaffer, E.; Krsk, R.; Braun, R. Microbiological degradation of deoxynivalenol and 3-acetyl-deoxynivalenol. In Mycotoxins and Phycotoxins: Developments in Chemistry, Toxicology and Food Safety; Miraglia, M., van Egmond, H.P., Brera, C., Gilber, J., Eds.; Alaken, Inc.: Fort Collins, CO, USA, 1998; pp. 279–285.
45. Schatzmayr, G.; Zehner, F.; Täubel, M.; Schatzmayr, D.; Klimitsch, A.; Loibner, A.P.; Binder, E.M. Microbiologicals for deactivating mycotoxins. Mol. Nutr. Food Res. 2006, 50, 543–551. [CrossRef]
46. European Food Safety Authority. Opinion of the Scientific Panel on additives and products or substances used in animal feed (FEEDAP) on the safety of the product “Biomin BBSH 797” for piglets, pigs for fattening and chickens for fattening. Efsa J. 2005, 3, 169. Available online: https://efsa.onlinelibrary.wiley.com/doi/abs/ 10.2903/j.efsa.2005.169 (accessed on 1 January 2020). [CrossRef]
47. Zhao, C.; Liu, G.; Li, X.; Guan, Y.; Wang, Y.; Yuan, X.; Sun, G.; Wang, Z.; Li, X. Inflammatory mechanism of rumenitis in dairy cows with subacute ruminal acidosis. Bmc Vet. Res. 2018, 14, 1–8. [CrossRef]
48. Reisinger, N.; Schürer-Waldheim, S.; Mayer, E.; Debevere, S.; Antonissen, G.; Sulyok, M.; Nagl, V. Mycotoxin occurrence in maize silage—A neglected risk for bovine gut health? Toxins 2019, 11, 577. [CrossRef]
49. Gallo, A.; Giuberti, G.; Frisvad, J.C.; Bertuzzi, T.; Nielsen, K.F. Review on mycotoxin issues in ruminants: occurrence in forages, effects of mycotoxin ingestion on health status and animal performance and Practical Strategies to counteract their negative effects. Toxins 2015, 7, 3057–3111. [CrossRef]
50. Kamyar, M.; Rawnduzi, P.; Studenik, C.R.; Kouri, K.; Lemmens-Gruber, R. Investigation of the electrophysiological properties of enniatins. Arch. Biochem. Biophys. 2004, 429, 215–223. [CrossRef]
51. Noroozian, E.; Lagerwerf, F.; Lingeman, H.; Brinkman, U.A.; Kerkhoff, M.A. Determination of roquefortine C in blue cheese using on-line column-switching liquid chromatography. J. Pharm. Biomed. Anal. 1999, 20, 611–619. [CrossRef]
52. Kopp, B.; Rehm, H.J. Antimicrobial action of roquefortine. Eur. J. Appl. Microbiol. Biotechnol. 1979, 6, 397–401. [CrossRef]
53. Bentley, R. Mycophenolic Acid: A One Hundred Year Odyssey from Antibiotic to Immunosuppressant. Chem. Rev. 2000, 100, 3801–3826. [CrossRef]
54. Hymery, N.; Mounier, J.; Coton, E. Effect of Penicillium roqueforti mycotoxins on Caco-2 cells: Acute and chronic exposure. Toxicol. Vitr. 2018, 48, 188–194. [CrossRef] [PubMed]
55. Haggblom, P. Isolation of roquefortine C from feed grain. Appl. Environ. Microbiol. 1990, 56, 2924–2926. [CrossRef]
56. Shier, W.T.; Shier, A.C.; Xie, W.; Mirocha, C.J. Structure-activity relationships for human estrogenic activity in zearalenone mycotoxins. Toxicon 2001, 39, 1435–1438. [CrossRef]
57. Abidin, Z.; Khatoon, A. Ruminal microflora, mycotoxin inactivation by ruminal microflora and conditions favouring mycotoxicosis in ruminants: A review. Int. J. Vet. Sci. 2012, 1, 37–44.
58. Valenta, H.; Vemmer, H. In vitro-Untersuchungen zum Metabolismus von Zearalenon bei Inkubation mit Pansensaft. In Proceedings of the 18 Mykotoxin-Workshop, Kulmbach, Germany, 10–12 June 1996; pp. 10–12. Available online: https://www.researchgate.net/publication/283891163_In_vitro-Untersuchungen_ zum_Metabolismus_von_Zearalenon_bei_Inkubation_mit_Pansensaft (accessed on 1 January 2020).
59. Kallela, K.; Vasenius, L. The effects of rumen fluid on the content of zearalenone in animal fodder. Nord. Vet. Med. 1982, 34, 336–339.
60. Coppock, R.; Mostrom, M.; Sparling, C.; Jacobsen, B.; Ross, S. Apparent zearalenone intoxication in a dairy herd from feeding spoiled acid-treated corn. Vet. Hum. Toxicol. 1990, 32, 246–248.
61. Bloomquist, C.; Davidson, J.; Pearson, E. Zearalenone toxicosis in prepubertal dairy heifers. J. Am. Vet. Med. Assoc. 1982, 180, 164–165.
62. Weaver, G.; Kurtz, H.; Behrens, J.; Robison, T.; Seguin, B.; Bates, F.; Mirocha, C. Effect of zearalenone on the fertility of virgin dairy heifers. Am. J. Vet. Res. 1986, 47, 1395–1397.
63. Vanyi, A.; Timar, I.; Szeky, A. Fusariotoxicoses. IX. The effect of F-2 fusariotoxin (zearalenone) on the spermatogenesis of rams and bulls. Magy. Allatorv. Lapja 1980, 35, 777–780.
64. Marczuk, J.; Obremski, K.; Lutnicki, K.; Gajecka,M.; Gajecki,M. Zearalenone and deoxynivalenol mycotoxicosis in dairy cattle herds. Pol. J. Vet. Sci. 2012, 15, 365–372. [CrossRef]
65. Schneweis, I.; Meyer, K.; Hormansdorfer, S.; Bauer, J. Mycophenolic acid in silage. Appl. Env. Microbiol. 2000, 66, 3639–3641. [CrossRef]
66. Hu, L.; Rychlik, M. Biosynthesis of 15N3-labeled enniatins and beauvericin and their application to stable isotope dilution assays. J. Agric. Food Chem. 2012, 60, 7129–7136. [CrossRef]
67. Jeyanathan, J.; Escobar, M.; Wallace, R.J.; Fievez, V.; Vlaeminck, B. Biohydrogenation of 22:6n-3 by Butyrivibrio proteoclasticus P18. Bmc Microbiol. 2016, 16, 1–12. [CrossRef]
68. Debevere, S.; De Baere, S.; Haesaert, G.; Rychlik, M.; Fievez, V.; Croubels, S. Development of an UPLC-MS/MS method for the analysis of mycotoxins in rumen fluid with and without maize silage emphasizes the importance of using matrix-matched calibration. Toxins 2019, 11, 519. [CrossRef]
69. European Commission. Commission Decision of 12 August 2002 implementing Council Directive 96/23/EC concerning the performances of analytical methods and the interpretation of results. Off. J. Eur. Communities 2002, L221, 8–36. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32002D0657 (accessed on 1 January 2020).
70. Knecht, J.; Stork, G. Prozentuales und logarithmisches verfahren zur berechnung von eichkurven. Z. Anal. Chem.1 1974, 270, 97–98. [CrossRef]
71. Heitzman, R.J. Veterinary Drug Residues. Residues in Food Producing Animals and Their Products: Reference Materials and Methods, 2nd ed.; Heitzman, R.J., Ed.; Blackwell Scientific Publications: Oxford, UK, 1992; ISBN 92-826-4095-7. Available online: http://aei.pitt.edu/44343/1/A7247.pdf (accessed on 1 January 2020).
72. Committee for Medicinal Products for Veterinary Use (CVMP). VICH Topic GL49: Studies to Evaluate the Metabolism and Residue Kinetics of Veterinary Drugs in Food Producing Animals: Validation of Analytical Methods Used in Residue Depletion Studies; European Medicine Agency: London, UK, 2015. Available online: https://www.ema.europa.eu/ en/vich-gl49-studies-evaluate-metabolism-residue-kinetics-veterinary-drugs-food-producing-animals (accessed on 1 January 2020).
73. Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [CrossRef]
74. Barton, K. MuMIn: Multi-Model Inference, Version 1.43.6. Available online: https://cran.r-project.org/web/ packages/MuMIn/MuMIn.pdf (accessed on 2 February 2020).
75. Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; pp. 1–258. ISBN 978-3-319-24277-4. Available online: http://ggplot2-book.org/index.html (accessed on 1 January 2020).







.jpg&w=3840&q=75)