Metal Propionates: A Boon for Today’s Dairy Animal
Published: May 29, 2012
By: Dr. Deepak Kumar Dubey (Kemin)
When we talk about dairy nutrition our discussion is limited to energy, proteins, fats, etc., which have a direct impact on animal health and milk production. As a result, the nutrients that require less quantity, for example minerals or more precisely trace minerals, are often forgotten.
Minerals are inorganic elements required for proper growth, body maintenance, nerve function, body enzymes and hormones. Trace minerals, as per the name, are required in a very small quantity but their consumption is essential. Trace minerals include: zinc, copper, cobalt, manganese, iodine, iron and chromium.
Even though minerals are essential for proper functioning of the body, they are not properly fed. The impact of mineral deficiency cannot be seen immediately. It may take time for clinical symptoms to appear but a decline in the performance of animals is quite common.
The very important factor in this regard is that mineral absorption is much less as compared to all other nutrients. Feed stuffs are usually deficient in minerals so animals are provided with supplementing mineral sources.
Trace Minerals - Absorption Matters
Just having the minerals present in the feed is one thing, but they must be absorbed from the feed in order to be utilized by the animal.
The pathway of absorption is a complex one. Obviously, we start with the intestines, down to the mucosa, then to individual villi, and then to individual cells.
Absorption of minerals must occur at the cellular level in order to be adequately absorbed by the animal.
Two Hypotheses Regarding Mechanism of Uptake
- Organic trace minerals are co-transported across membranes (mineral + carrier molecule).
- Organic trace minerals are absorbed independently of organic moiety (mineral in ionic form) by specific carrier proteins.
Based on the first hypothesis, organic trace minerals are thought to be co-transported across the membrane with a carrier molecule. In this case, the carrier molecule is essential amino acids.
It was well known that cells are highly selective towards absorption of essential amino acids, such as methionine. So, it is thought that by binding methionine to zinc, the zinc was carried along with the methionine, which neutralized the charges across the membrane.
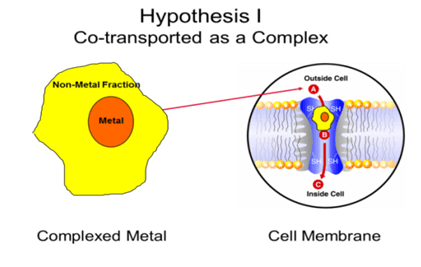
If Hypothesis I is correct, the mineral would be co-transported as a complex across the cell membrane. That would mean that the metal, along with the non-metal fraction which it is associated with, would be absorbed as that complex, and not be ionized (dissociated) before cellular absorption. The cell would need to have a transport mechanism, or various transport systems, that would allow uptake of different minerals as complexes, not as ions.
Hypothesis II (Kemin´s Hypothesis) is that organic minerals are absorbed independently of organic moiety, and that this absorption phenomenon was due to proteins imbedded in the cellular membrane; not due to some carrier molecule.
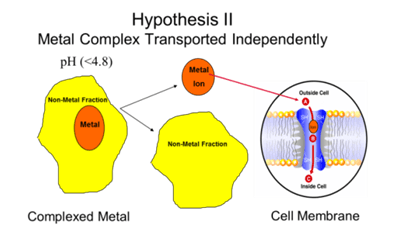
The second hypothesis is quite different. In this particular case, as soon as the metal complex arrives in the gut, it is dissociated into the zinc ion and the methionine ion. Here, the zinc itself is absorbed through particular carrier proteins that are embedded in the cell. These carrier proteins have within them the ability to effectively neutralize the charge and carry it through the cell membrane. This is all designated in the diagram seen here (Figure. 2)
You will note in the mechanism through which zinc is absorbed by the zinc binding protein, that the complex goes through a process we call chelation. This process is energy dependent through the utilization of ATP for the transport, and then the zinc is successfully transported to the other side of the cell membrane.
Proof of Hypothesis II
Research: Study of Zn Uptake by Cell
Scientist: Kathleen Beutler, David Brautigan and O. Pankewycz,
Place of Study: Center for Cell Signaling, University of Virginia, Charlottesville, Virginia
An in vitro, bound complex experiment, using cell culture models, was used to test whether cells actually utilized organic minerals.
The cells that were utilized were either kidney fibroblasts or intestinal epithelial cells.
Importantly, zinc methionine was used as the test material to see if it absorbed through one of the two hypotheses. The zinc methionine used was synthesized with two radioactive labeled molecules: zinc-65 and methionine-35. It is important to note that we can put two different radioactive labeled molecules on the same molecule. The beauty of this is that if the molecules dissociate, they may be absorbed at different rates. If they don´t dissociate, obviously they will be absorbed at the same rate. In order to make certain that the cells would be able to absorb both zinc and methionine, they were artificially starved for these two nutrients before the experiment began.
At the time of the experiment, a large amount of non-radioactive methionine was also added to the petri dish.
(Figure 3: Results)
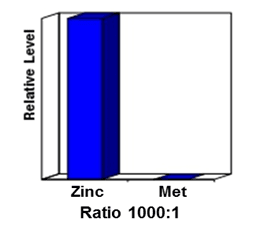
In this case, the ratio was 1,000 radioactive zinc to 1 radioactive methionine. This was significantly different than the 1 to 1 ratio we would expect if they were co-transported. Therefore, this study proves that zinc is not co-transported across the cell in its organic moiety, but rather indicates that zinc is transported as a metal ion. Based on this study, further trials have also confirmed this method of mineral transport.
Inference of the Organic Mineral Absorption
Zn-dipropionate <-----> Zn+2 + 2 Propionate
Zn-methionine <-----> Zn+2 + Methionine
Basically, this means that trace minerals must first solubilize (dissolve in water), but then must also dissociate (break apart) before they can be absorbed. An example of this is zinc propionate, which must first dissociate to form a separate zinc ion and propionate before transport of ionic zinc by the cell.
Need for Organic Minerals
What are the sources of zinc?
- zinc oxide
- zinc sulfate
Both are inexpensive and non-organic sources.
Then of course there are the organics, i.e. zinc propionate and other chelates.
What is the difference between the inorganic and the organic, and why is this important? What is the difference in cost between the two groups? The organics are 2 - 4 times more expensive than the non-organics. So you have to ask the question, why would anyone utilize the organic sources? It is all about relative bioavailability.
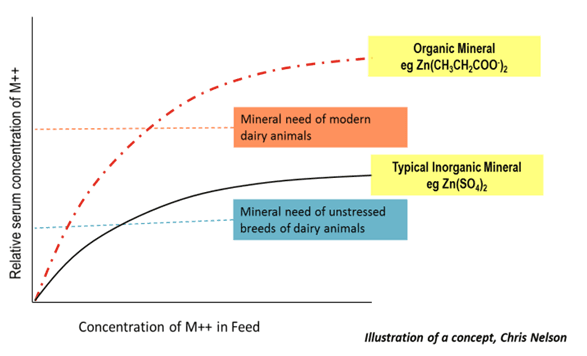
When we look at zinc sulfate, you see that we can add more and more zinc sulfate to the feed, but at certain point the total amount absorbed plateaus and no more zinc can be absorbed. Absorption of zinc propionate also plateaus, but is significantly higher. Why is that important? If we imagine that the animal is in a low stress situation, and the low stress indicator on the graph represents the amount of zinc they need in their blood stream, we can see it is possible to reach that level with both zinc sulfate and zinc propionate. However, when in production conditions, as indicated on the graph, the amount of zinc that the animal requires is impossible to reach with zinc sulfate. You have to use organic zinc, i.e. zinc propionate.
Relative Bioavailability (%) – Inorganic Zinc versus Zinc Propionates
A zinc bioavailability trial was conducted in order to elicit the relative bioavailability of zinc propionate. Baby chicks were used because they can be completely depleted of zinc and other trace minerals. The basal diet consisted of 12.4 ppm zinc.
Treatment groups consisted of zinc supplementation from zinc sulfate and zinc propionate at multiple dose rates of 10 and 20 ppm of added zinc. Data was collected after 21 days and plotted using a regression analysis.
Figure 5. Source: (1995). Bioavailability of zinc from organic and inorganic sources using the chick bioassay technique. (Internal publication, BB-03-00234)
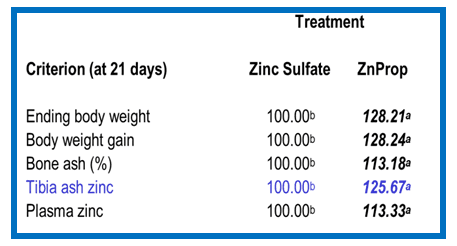
This table helps to show the biological availability of zinc propionate relative to zinc sulfate. When looking at the data, the values generated by zinc sulfate were assigned a value of 100. In all of the parameters shown here, zinc propionate was significantly more bioavailable than zinc sulfate.
Bioavailability of Zinc Sources in Cows
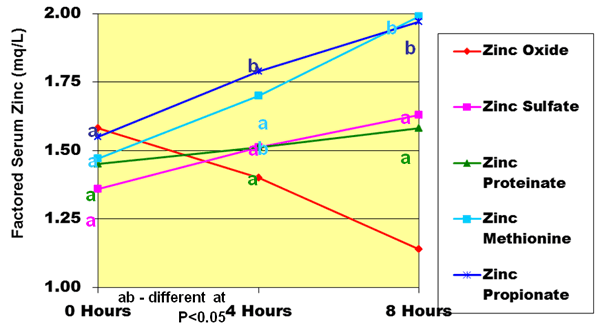
The bioavailability of zinc in ruminants is also a critical issue. A comparative analysis of various zinc sources in dairy cows was conducted at the Miner Institute in Chazy, New York. Twenty-one Holstein cows were fed minerals to provide 300 ppm of supplemental zinc. The zinc sources compared were zinc oxide, zinc sulfate, zinc proteinate, zinc methionine and zinc propionate. There were four cows per treatment, except for the zinc oxide treatment, which only contained one cow. A preliminary trial was conducted which basically revealed that the zinc from zinc oxide was highly unavailable, thus, there was less interest in looking at zinc oxide as a treatment. Plasma serum zinc concentrations were measured at 0, 4 and 8 hours post-bolusing for three days.
Figure 6. (Studies of C.S. Ballard, C.J. Sniffen and Larry Schlatter, Miner Institute, Chazy, NY)
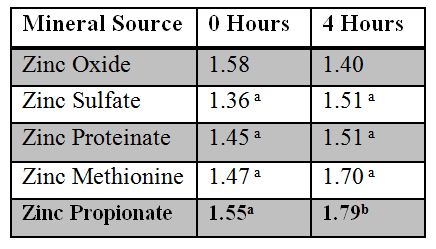
Figure 7. Plasma serum zinc concentration of various Zinc Sources
Conclusion: Why metal Propionates are better?
Basically, organic trace minerals solubilize and dissociate at the desired place in the digestive tract.
When compared to metal propionates, other mineral sources may:
- Not completely solubilize. A mineral must become soluble before it can readily dissociate, and minerals with low solubility, such as metal oxides, have a relatively small chance for eventual cellular uptake.
- Some mineral sources may dissociate too rapidly. If too much of a mineral dissociates in the rumen or before reaching the intestinal binding sites, the mineral may be re-complied to form tightly bound complexes that may simply wash through the system without the mineral being utilized.
- A mineral may also dissociate too late. Some organic minerals that are too tightly chelated may not have a very high rate of dissociation prior to reaching the absorption sites.
Metal propionate minerals are highly soluble, and dissociate at a rate that is approximately equal to the need or availability of the respective metal transport sites throughout the GI tract. If this did not occur, you would not measure increased bioavailability in the animal. Therefore, there is not a "flood" of ionized mineral at a given point in the system, but rather an appropriate supply of mineral made available where it is needed and can be utilized the most.
KemTRACE®: A Blend of Metal Propionates
Kemin has developed a unique combination of trace minerals containing essential metal propionates in the form of a product called KemTRACE® Star. It contains zinc, copper, cobalt, maganese, iron, iodine and chromium as per NRC guidelines. It´s proven to be highly bioavailable and provides better return on investment in today´s production systems.
Related topics:
Authors:
Kemin Industries, Inc
Recommend
Comment
Share
Zoetis
30 de mayo de 2012
Good artcile about Metal Propionates for Dairy Animal , very informative , Keep it up Deepak
Recommend
Reply
29 de mayo de 2012
a good article about Metal Propionates for Dairy Animal . Wood wishes for the vAuthorhich is thought provoking.
Good wishes for the Author.
Recommend
Reply

Would you like to discuss another topic? Create a new post to engage with experts in the community.










