Introduction
Sugars are rapidly and extensively fermented in the rumen. Clearly, adding sugar to a diet already high in ruminally degraded carbohydrates should offer little benefit and could decrease digestibility of fiber, whereas diets that have less-than-optimal rumen degraded carbohydrate probably will benefit the most from addition of sugars. Therefore, dietary situations influence the optimum feeding rate of between 2.5 and 5% supplemental sugar (Broderick and Radloff, 2004; Firkins et al., 2008b). The typical inference is of a double-edged sword in that sugars provide a burst of energy to "jump start" ruminal processes, but excess sugar intake could cause a burst of acid production that promotes acidosis. However, current research complicates these traditional interpretations and adds a different dimension to consider.
Nearly all models for ration evaluation or description of rumen function work on a daily interval even though the cow eats multiple meals of varying amounts of feed per meal. They ignore that, in a group situation, there is a large variation among cows and even by the same cow over multiple days. Fiber sources are typically degraded at about 5%/hour, thus taking about one day to degrade. Starches are degraded and fermented in the 10 to 20%/hour range, thus taking several hours to degrade and providing a more continuous supply of sugars over the day (among all meals). When sugars are released by polysaccharide hydrolysis or by feeding them directly, they are fermented within an hour (i.e., they have > 100%/hour fermentation rates). The more frequent the meals are consumed, the more this daily composite of dietary carbohydrate sources is divided into smaller increments, thereby decreasing the opportunity to "jump-start" microbial function. We should be formulating diets that have a proper ratio of rumen-degraded carbohydrate relative to effective fiber (Zebeli et al., 2010) and then fine-tuning this concept according to different farms´ forage and grain sources and managerial capacities. TMR feeding and enhancing multiple meals per day through multiple feedings or pushups should reduce justification for sugars from this vantage.
If jump-starting is less important, then why would addition of moderate amounts of sugars potentially increase ruminal fiber digestibility (Broderick and Radloff, 2004)? Growing evidence supports the concept that there is a core population of highly specific particle-associated bacteria that efficiently degrade fiber, whereas there is considerable variation among loosely associated or fluid-associated bacterial populations that secondarily break down partially degraded fibers and non-structural carbohydrates (Wallace, 2008; Welkie et al., 2010). Sugar fermenters can provide growth factors or help control the fluid environment that bathes the adherent fibrolytics. Optimizing the use of sugars probably depends on how well we can predict ruminal carbohydrate degradability and manage meal feeding behavior in groups of cows on farms with varying grain and forage sources, which is why I will address rumen microbial ecology.
Although a low ratio of effective fiber to rumen-degraded carbohydrate has long been associated with milk fat depression, in most studies sugar addition had neutral effects on or actually supported a higher production of milk fat (see below). In order to understand when and how sugars could increase milk fat yield, we must address how sugars can support a more consistent dry matter intake (DMI) and can influence biohydrogenation of unsaturated fat. Enhanced DMI within a group of cows obviously increases NEL intake needed for production of energy-corrected milk (including milk fat), but DMI also is the most critical driving variable positively related to microbial protein synthesis (Oldick et al., 1999) and milk protein yield when cows are fed supplemental fat (Wu and Huber, 1994). Milk fat yield is strongly influenced by ruminal production of trans fatty acids that can pass to the intestine and depress milk fat secretion (Jenkins et al., 2008). Either directly (through microbial action) or indirectly (through particle sorting), inclusion of dietary sugars can interact with the source and particle length of forage that is consumed by the cow to be "effective". My objectives are to integrate the ramifications of these factors when considering sources of sugars in dairy rations.
Lactic Acid Production Versus Accumulation
We have all been taught in our ruminant nutrition classes that lactic acidosis is the scourge of concentrate feeding for beef and dairy cattle. Excess amount or rate of concentrate consumption favors lactate-producing Streptococcus bovis, the "weed of the rumen". Although it can metabolize glucose to lactate at one-half the ATP yield per molecule of glucose, it can still metabolize glucose to lactate at least 5 times faster and yield more ATP per unit of time than to volatile fatty acids (VFA). Lactic acid is 10-fold more acidic than the VFA per molecule, so the low pH tolerance of S. bovis allows it to outcompete the resident lactate consumers such that while increasing lactate concentration is decreasing the pH, the decreasing pH is further increasing lactate concentration. After further cycling, eventually S. bovis is replaced by lactobacilli, further exacerbating the cycle. Lactate is produced in L and D forms, but the conversion of D to L is very slow, so the buildup of D-lactate in the blood causes acute systemic acidosis. Although Nagaraja and Titgemeyer (2007) document these findings for cattle with acute acidosis, they also explain why subacute rumen acidosis (SARA) leads to many problems in feedlot cattle even though lactate concentration in the rumen (and blood) remains only briefly increased and then only peaks at about 5 mM (< 5% of total organic acids). Moreover, induction of acute acidosis only consistently raised lactate concentration when wheat was used, whereas induction of acute acidosis with corn or beet pulp elevated butyrate and propionate, respectively (Lettat et al., 2010).
Grain-induced SARA was associated with reduced bacterial diversity and increased occurrence of E. coli phylotypes (Khafipour et al., 2009). As discussed below, a resilient bacterial community should have many redundant fluid-associated bacterial groups, including those that utilize lactic acid. These Canadian researchers recently steeped barley grain with an equal quantity of water or water plus 0.5% lactic acid. Although Iqbal et al. (2009) described how lactic acid decreased starch digestibility statistically, the difference in effective degradability was trivial (< 1%). Thus, the increase in milk fat% resulting from the lactic acid steeping was arguably most physiologically related to a significantly decreased immune response from Gramnegative bacteria such as E. coli (Iqbal et al., 2010). The same researchers have linked bursts of serum virulence factors from E. coli in dairy cattle with grain-induced SARA (Khafipour et al., 2011). Although E. coli would be considered a minor player based on its low abundance, fluctuating bursts could augment important systemic responses. They could increase and suddenly die as pH lowers; their lysis leaves behind lipopolysaccharide cell wall fragments that can pass through the rumen epithelial membrane (Emmanuel et al., 2007) but not necessarily into blood (Gozho et al., 2007) to trigger a host immune response. To prepare the cow´s system for an expected bacterial infection, the system would prioritize energy use for the immune response and thereby decrease fatty acid synthesis in the mammary gland (Zebeli and Ametaj, 2009). Thus, it is not the production or even the long-term accumulation of lactate that is the problem; the cow´s rumen microbial population helps to prevent a disruption in her normal rumen function unless the normal "good" bacteria are intermittently challenged by undesirable bacteria such as certain phylotypes of E. coli.
The rumen contains a variety of lactilytic bacteria and entodiniomorphid protozoa most of which do not use lactate as their main energy source (Nagaraja and Titgemeyer, 2007). These authors also explained how entodiniomorphid protozoa can store cache dietary starch and isotrichid protozoa can convert dietary sugars to stored glycogen to help "buffer" the rumen unless a lowered pH inhibits them. Organic acids (Martin, 1998), direct-fed microbials (Martin and Nisbet, 1992), and even residual fermentation extract in distillers byproducts (Fron et al., 1996) can enhance the lactilytic populations of the rumen to help prevent a rapid pH decline in the rumen. As lactate production increases, though, strains of Megasphaera elsdenii are probably the most well known to shift from glucose to lactate as substrate such that S. bovis rarely increases in abundance (Nagaraja and Titgemeyer, 2007). Henning et al. (2010) selected M. elsdenii strains as potential probiotics for beef cattle to reduce SARA during the shift from high forage to higher grain diets and showed that the treatment improved pH control and concurrently increased molar proportion of butyrate. In a previous study (Klieve et al., 2003), a M. elsdenii probiotic established at functional abundance in beef cattle, but when given enough time to adapt to a shift from a forage- to a grain-based diet, the resident M. elsdenii populations in the control group lagged but increased to the same abundance. Additives therefore probably do not necessarily increase the abundance in the longer term, but reduce the transition time needed to increase the abundance of the lactilytic populations. We would target the obvious transition phase from a dry cow diet to a lactation ration for such a role, but any feeding situation that promotes variation among cows (e.g., overcrowding) and variation among diets (e.g., forage sorting) promotes intermittent patterns of microbial transition that could lead to unexpected SARA or at least milk fat depression. Although not as well studied as with additives, addition of sugars probably also increase the lactilytic populations and thereby helps to buffer against transient bursts of opportunistic sugar fermenters.
Sugars, Ruminal pH, and Fiber Digestibility
We want to efficiently degrade particulate matter before it passes from the rumen; therefore, we must optimize bacterial colonization of stems, leaves, and even starch granules. Because increasing grain ingestion usually decreases ruminal pH, Calsamiglia et al. (2008) varied dietary forage:concentrate ratios while maintaining different constant pH values in continuous culture. Adding acid to systematically decrease pH had a direct effect that was dose-responsive and predictive for many variables, and was much more critical than forage:concentrate. However, while confirming the important response to low pH, these pH values were maintained constantly and were uniformly distributed throughout the entire flasks by rapid stirring of the pH-controlled buffer with finely ground feed particles. In contrast, pH has been long known to depend on the time after feeding, particle size, and location of those particles stratified and slowly mixed in the rumen. Thus, this in vitro model does not consider how variable pH influences the initial attachment and progressive need for growth factors in the rumen´s larger feed particles containing substrate that is more limited by surface area.
The primary cellulolytic bacteria, which also contribute to hemicellulose degradation, are less tolerant to a low pH. If we add too much grain to the diet, fiber digestibility can be reduced because of lower pH. This decreased fiber digestibility is important because it could promote bulk fill limitation of voluntary feed intake. Low ruminal pH could reduce binding by cellulolytics to particulate matter in the rumen, allowing more acid-tolerant bacteria to initially adhere and therefore outcompete the highly efficient fibrolytics for attachment sites on newly ingested feed particles (Mouriño et al., 2001). After initial adhesion, bacteria must grow (divide into many more cells) to rapidly and extensively degrade that particle before passage, which is very rapid in a high producing cow. Although we often focus on ruminal pH < 6.0 as the main limitation of fiber digestibility, more current work with molecular techniques has shown that even cows with pH < 6.0 can maintain normal populations of cellulolytic bacteria (Palmonari et al., 2010). Even grain-induced acidosis did not reduce the abundance of cellulolytic bacteria unless it progressed to what was classified as severe acidosis (Khafipour et al., 2009).
In studies investigating the "carbohydrate effect" independent of pH (Piwonka and Firkins, 1996), we discussed the potential for higher glucose supply to reduce fiber degradation through production of proteinaceous inhibitors. Although these inhibitors could be detrimental in high grain diets, what if some proteinaceous inhibitors provide some benefit in diets with moderate amounts of grain? Some of these proteins inhibit the low abundance/high activity group termed "hyperammonia-producing bacteria", which are being targeted by various additives to improve efficiency of protein usage (Calsamiglia et al., 2007). Unlike the amylolytic bacteria that have a moderate rate of deamination and that use the carbon skeletons for only a portion of their energy, these obligate amino acid fermenters rapidly reduce the availability of amino acids to stimulate growth of amylolytic or cellulolytic bacteria. Those bacteria that have been cultivated are inhibited by low pH, but a computer modeling study suggested a more direct antagonism by carbohydrate-fermenting bacteria against the hyper-ammonia producers (Rychlik and Russell, 2000). Thus, moderate provision of grain or addition of supplemental sugars could be limiting these obligate deaminators and maintaining a more consistent concentration of peptides and amino acids between meals to maintain a more efficient microbial ecosystem involved in fiber degradation.
Many, but not all, studies with sugars show increased molar proportion of butyrate or valerate (Heldt et al., 1999). A recent molecular analysis of rumen contents from feedlot beef steers (not fed sugars) documented that those with improved feed efficiency had increased butyrate and valerate concentrations (Guan et al., 2008). These VFA are much more important fuel sources for the rumen epithelium than are acetate or propionate (Kristensen, 2005). For sheep fed the same acidosis challenge diets, some sheep had increased rates of VFA absorption in vitro from rumen epithelia samples than other sheep (Penner et al., 2009), indicating that those sheep that had better protection against low pH also had faster absorption rates of VFA (plus their associated protons). Dr. Penner´s paper in this symposium should help explain the large among-cow variability in ruminal pH regulation. The main point stressed here is that sugar-fermenting, fluid-associated bacteria can help stimulate VFA absorption and thereby help resist a decline in ruminal pH.
In our study (Oelker et al., 2009) and several others we cited, ruminal pH was not decreased by sources of sugars in the diet. Therefore, so long as we maintain a proper environment for microbial populations, feeding less than 5% sugars rarely decreases ruminal pH and sometimes actually increases it. When 5 cows each were fed a control or a diet with 4.7% sucrose starting on the day after calving, the ruminal pH actually tended (P = 0.08) to be higher for the sucrose diet, and the time at which pH was below a 5.8 was numerically (P = 0.13) reduced by about 2.5 hours per day (Penner and Oba, 2009). If sugars reduce the time when pH is below a critical threshold and if initially low pH residually limits degradation of newly colonized fiber particles (Mouriño et al., 2001), then there should be efficient colonization of fiber particles ingested after each meal, assuming growth factors are not limiting. Broderick and Radloff (2004) provided evidence from two trials as well as from other studies that a moderate amount of sugars in the diet can increase NDF digestibility in dairy cattle.
Sugars and Rumen-Degraded Protein
The ability to consume lactate and stimulate fiber digestibility probably depends on having adequate rumen-degraded protein (RDP). Both low pH (Calsamiglia et al., 2008) and low nitrogen per se (Griswold et al., 2003) can decrease proteolysis by bacteria in continuous culture. A comprehensive metagenomics study confirmed the importance of M. elsdenii and various Prevotella species to help maintain a healthy rumen (Khafipour et al., 2009). The authors discussed the potential for a Prevotella probiotic to reduce the incidence of subacute acidosis. This diverse phylum is well documented for its starch-degrading and sugar-fermenting niche but also its major capacity to degrade proteins (Walker et al., 2005). Their active protease capacity funnels short peptides and amino acids to other bacteria for uptake and use for protein synthesis or for catabolism. Some strains of M. elsdenii have rapid rates of deamination, but not necessarily high proteolytic activity, and they likely use these amino acids more for energy after glucose or lactate become limiting (Rychlik et al., 2002). However, as explained by those authors, M. elsdenii produces branched chain VFA from their corresponding amino acids, which are required by most of the fibrolytic bacteria. When beef cattle were dosed with pure starch or glucose, lactate only spiked after feeding glucose, and there was a corresponding prolonged butyrate concentration, indicative of a stimulation of a butyrate-producing population (Arroquy et al., 2004b). In a companion study, lactate concentration again spiked when glucose was dosed at an equivalent concentration as starch, but lactate concentration decreased incrementally back to baseline with increasing supply of RDP (Arroquy et al., 2004a). Thus, having adequate RDP might be a prerequisite for allowing the sugar-fermenting/lactate-fermenting populations to "buffer" the ruminal fermentation from bursts of low pH, with the caveat that adding RDP would only be beneficial if peptide concentration is indeed limiting.
Research with isotopically labeled amino acids supports their stimulation of fiber digestibility (Walker et al., 2005). Specialist fibrolytics efficiently degrade native cellulose or hemicellulose, but many generalists can scavenge the oligosaccharides from enzymatic hydrolysis while supplying growth factors (including but not limited to branched chain VFA) to the fibrolytics. Colonization of both groups is extensively committed within 5 to 15 min and thereafter is a function of subsequent bacterial growth (Edwards et al., 2007). Moreover, protease activity has been proposed to expose more surface area to stimulate rate of fiber degradation (Colombatto and Beauchemin, 2009). Thus, maintaining proper RDP should allow proteolytic bacteria to support fiber degraders, whereas over-feeding grain could negate this benefit by reducing pH and/or the availability of growth factors.
Rumen-degraded protein does not just provide peptides for bacterial growth; it also is continually degraded to ammonia, the main N source for cellulolytic bacteria. In our study (Oelker et al., 2009) and several others we cited, adding molasses decreased ruminal ammonia concentration. Adding urea to corn silage diets recovered ammonia concentration to that of the control, but when we fed alfalfa hay (high in RDP), molasses did not influence ammonia concentration. The linear decrease in ammonia concentration with increasing sucrose substitution for starch without an increase in microbial N production is consistent with these responses (Broderick et al., 2008). The net concentration of ruminal ammonia depends on its production from RDP and transfer from blood urea N relative to its incorporation into microbial protein. Thus, a net decrease in ammonia concentration can be a sign of more efficient microbial protein synthesis that could be limited by peptide supply, not necessarily ammonia. If we can better understand how sugars influence ammonia production and uptake, we should have a greater opportunity to optimize the conversion of RDP into microbial protein with less need for extra RDP safety factors that promote excessive loss of N in the urine.
Many modeling efforts have focused on the three well characterized cellulolytic isolates: Fibrobacter succinogenes, Ruminococcus flavefaciens and R. albus. All require branched chain VFA and ammonia as the principal nitrogen source. Because branched-chain VFA are less likely to be limiting for their growth in the rumen compared with ammonia, the CNCPS and CPM models focus on providing adequate ammonia concentration for the cellulolytic populations but focus on peptide supply for the amylolytic bacteria. However, those authors have focused on the use of pure cultures and have typically ignored that these cellulolytics work in a consortium and benefit from preformed amino acids (Walker et al., 2005). The lack of interaction between the nonstructural carbohydrate bacteria and the structural carbohydrate bacteria in CNCPS/CPM might require more empirical consideration or adding constraints to optimize the integration of efficiency of RDP with ruminal carbohydrate degradability and source.
An alternative to fostering enzymatic attack within the rumen is to first expose feed to exogenous enzymes, especially those with fibrolytic activity (Beauchemin et al., 2003). However, even addition of exogenous amylase with insignificant activities against protein or fiber still stimulated NDF digestibility (Kingerman et al., 2009). The authors referenced a proposed mechanism that amylase stimulated non-cellulolytics to cross-feed with cellulolytic bacteria. These results with amylase treatment are in contrast with meta analyses showing decreased ruminal NDF digestibility with increasing starch concentration (Firkins et al., 2001; Nousiainen et al., 2009). Thus, a small amount of sugars could stimulate an optimal development/progression of the consortium needed for efficient degradation of fiber in diets with moderate starch concentration so long as there is adequate RDP for the consortium.
Both depressed pH and increasing concentrate proportion in diets are well known to potentially depress milk fat, sometimes sporadically among different cows fed the same diet and with shifts in bacterial populations (Weimer et al., 2010). To sort out some of these responses, continuous culture has been used to establish conditions to try to better document a mechanism. Continuously decreasing pH to 5.6 in continuous culture dramatically elevated the outflow of the 18:1 trans-10 isomer that is strongly correlated with milk fat depression, and quantitative PCR analysis of biohydrogenating bacterial groups suggested a change in the 18:1 trans-producing group (Fuentes et al., 2009). Because pH was maintained at 6.2 in another continuous culture study, the linear decrease in biohydrogenation associated with increased sucrose addition was attributed to shifts in bacterial populations (Ribeiro et al., 2005). Future efforts should separate the rates of lipolysis from the kinetics of biohydrogenation (Jenkins et al., 2008). In addition to a role in lipolysis, rumen protozoa preferentially incorporate unsaturated fatty acids or biohydrogenation intermediates such as conjugated linoleic acids into their membranes, potentially reducing the risk for milk fat depression (Firkins et al., 2008a). Protozoal populations were changed by molasses and interacted with forage source (Oelker et al., 2009). In that paper, we noted that entodiniomorphid protozoa are prominent starch and fiber degraders but also can metabolize sucrose.
Sugars in Moderate Starch Diets
After surveying numerous studies with sugars, I have concluded that they are most efficacious when they can stimulate DMI (Firkins et al., 2008b). Propionate is the principal gluconeogenic precursor for glucose production, which occurs in the kidney but primarily in the liver. I subscribe to the theory that glucose demand by the mammary gland for milk synthesis directs liver production of glucose (Lesmosquet et al., 2009). That is, as the cow produces more milk volume (and lactose), this increases the need for the liver to supply that glucose; in contrast, increasing glucose supply by the liver will not necessarily increase production of milk lactose. Therefore, when propionate supply to the liver exceeds its needs for glucose release to the mammary gland, the propionate then can send a feedback loop to reduce feed intake (Allen et al., 2009). As stated previously, sugars tend to increase the ruminal concentration of butyrate and sometimes valerate, but typically not propionate (Heldt et al., 1999; Ribeiro et al., 2005). When purified corn starch was replaced with sucrose, propionate or butyrate concentrations were not affected, but valerate and the branched chain VFA plateaued at an intermediate sugar concentration (P < 0.05 quadratic response; Broderick et al., 2008). As the authors discussed, acetate and propionate condense to produce valerate; thus, sugars could also help suppress the net propionate absorption even if for a limited degree. Both DMI and milk fat yield increased linearly (P < 0.05) with increasing sucrose substitution for starch. In contrast with feeding sugars, increasing supply of highly degraded starch sources should increase propionate production (Firkins et al., 2006).
The relationship between nonstructural carbohydrates and milk fat yield probably depends on forage source. Recently, Weiss et al. (2009) documented an important interaction between dietary starch concentration and the ratio of alfalfa silage:corn silage for milk fat yield. With increasing alfalfa silage:corn silage, increasing dietary starch concentration tended to increase milk fat yield. However, with decreasing ratio of alfalfa silage:corn silage (i.e., more corn silage), increasing dietary starch above about 25% was associated with decreasing milk fat yield. This relationship to forage source is consistent with the greater potential to depress milk fat when Rumensin is added to high starch diets in which corn silage is the main forage (Oelker et al., 2009). In the study of Penner and Oba (2009), replacing corn grain with sucrose increased DMI (P < 0.05) and milk fat yield (P < 0.10) and decreased (P < 0.05) the concentration of the 18:1 trans-10 fatty acid isomer that is strongly associated with milk fat depression. I am not suggesting that adding sugars can prevent milk fat depression in diets with excess fermentable carbohydrate; in fact, sugars seem to work better in lower starch diets (Firkins et al., 2008b). Still, sugars should not increase the risk of milk fat depression and could be reducing this risk if kept at appropriate levels (< 5% added sugar).
Whey provides a moderate amount of RDP and is a good source of lactose, which is extensively used by ruminal microbes. Consistent with previous discussion,
feeding whey to replace starch increased DMI, milk fat % (with a numerical trend for increased yield), and butyrate percentage (Charbonneau et al., 2006). This expected increase in lactose fermentation to butyrate was proposed to increase conversion of butyrate to beta-hydroxybutyrate (BHBA) for fuel in ruminal papillae, whereas the released BHBA was suggested to stimulate hepatic gluconeogenesis from propionate and reduce hepatic lipid infiltration in transition cows (DeFrain et al., 2006). Butyrate´s role in papillae regeneration for increased surface area and faster VFA absorption for transition cows needs further corroboration (Penner and Oba, 2009). The latter authors discussed a potential role for increased passage rate with sugars, but increasing ruminal motility also can increase VFA absorption because of increased diffusion to the rumen epithelium (Storm and Kristensen, 2010). The classical work of Palmquist et al. (1969) demonstrated that BHBA only contributes carbon for the priming unit (the first four carbons) of synthesized fatty acids; because acetate is needed for subsequent fatty acid elongation, BHBA contributed a maximum of 8% of the fatty acid carbon. Despite several references to this role for butyrate, increased BHBA supply to the mammary gland should not provide a direct explanation for the increased milk fat often associated with feeding sugars.
Sugars and Feedbunk Management
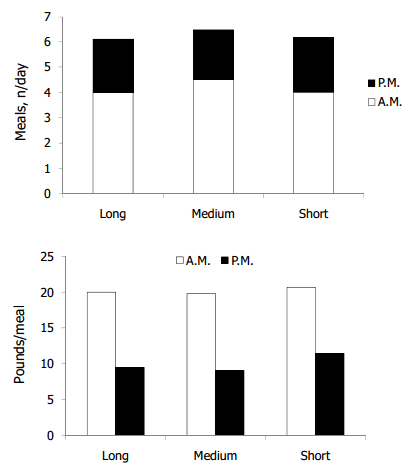
We are all acutely aware of the need for optimize the amount of energy intake through concentrates containing starch (as diluted by fibrous byproducts) while maintaining at least a minimum amount of forage NDF. I will refer readers to a recent article relating physically effective NDF (peNDF) to rumen-degraded starch percentages for useful application of the Penn State separator to optimize this ratio to at least 1.45:1 (Zebeli et al., 2010). Moreover, based on the previous discussion, I will stress that diets primarily composed of corn silage as the forage sources are more likely to cause milk fat depression. Zebeli et al. (2009) chopped corn silage (kernel processed) to three theoretical lengths of cut (approximately 0.55, 0.32, and 0.22 inches, respectively; Figure 1). Particle length was related quadratically (P = 0.08) to number of meals per day but linearly (P = 0.09) to the amount of feed consumed per meal. The diets were mixed as TMR and offered at 7:30 a.m. In the daytime, cows consumed more of the TMR, and the medium-sized silage had the highest number of meals (explaining the quadratic response). However, during the evening, there was no difference in meal numbers, but the cows fed the corn silage with the shortest particle length still consumed more feed per meal. Thus, the coarsest corn silage probably limited (P < 0.05) DMI from bulk fill (45.1 lb/day) compared with the other two treatments; those fed the medium silage increased DMI (48.0 lb/day) because, on average, they ate more meals, but those fed the short silage (48.4 lb/day) ate more feed per meal, primarily in the evening. As particle length of corn silage increased, cows linearly increased (P < 0.01) their preference for particles retained on the 1.18-mm screen as well as those particles passing through that screen but recovered in the pan.
In the study of Penner and Oba (2009), adding dry sucrose promoted selection for the particles recovered in the pan (presumably with a higher concentration of added dry sucrose), especially in the first week after calving. In contrast, in our study (Oelker et al., 2009), adding liquid molasses to the corn silage-based diet increased measured particle size because small particles were conglomerated and therefore not recovered in the pan. Although these were individually fed cows, molasses seemed to reduce sorting behavior in corn silage diets but not in alfalfa hay diets; we attributed the latter response to adding water to the dry hay TMR immediately prior to mixing in liquid molasses (which would not coat the wet alfalfa hay particles). In contrast with their hypothesis (and the prevailing wisdom), a recent report suggested that water addition to haylage/corn silage diets actually increased sorting behavior (Miller-Cushon and DeVries, 2009). In our study, we anecdotally noted that water softened dry hay particles and consistently improved DMI, and I would suspect a similar response in field conditions, but it does not automatically follow that water will reduce sorting against long particles for all types of diets. When applied to the TMR in place of water, liquid feeds should reduce sorting for small particles and against long particles, decrease slug feeding of grain, and provide an additive benefit to the effect of sugars per se on the ruminal fermentation. Sugars in liquid feeds could allow the use of slightly coarser corn silage to help reduce milk fat depression with less risk of this response being partially negated by cows sorting, especially in group situations. Moreover, in addition to a more consistent intake of forages and grain from reduced sorting, sugars make diets more acceptable to dairy cows (Murphy et al., 1997). Even if nutrient demand by the mammary gland is driving total voluntary DMI, greater acceptability could be attracting cows back to the feedbunk more frequently and thereby spacing out the inter-meal frequency. More work is needed to verify this contention.
OSU Production Responses with Liquid Feeds
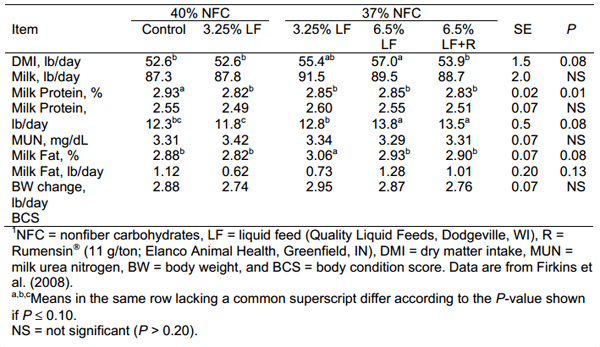
We integrated three lactation trials with individually fed Holstein cows fed molasses-based liquid feeds containing different non-protein nitrogen and fat sources (Firkins et al., 2008b). When liquid feed was added to diets containing 40% non-fiber carbohydrate (NFC), DMI and milk production were not affected (Table 1). However, when NFC was reduced to 37%, milk fat yield increased (P < 0.08) and DMI tended to increase when liquid feed was added. When doubling the inclusion rate of liquid feed, DMI increased compared with control unless Rumensin was added. As in many other cases, Rumensin maintained milk fat yield with decreased DMI, and the combination of Rumensin and liquid feed did not depress milk fat secretion. Another trial in that publication showed that adding liquid feeds as the last ingredient of the TMR maintained DMI even when fat also was added, and the combination increased milk fat production.
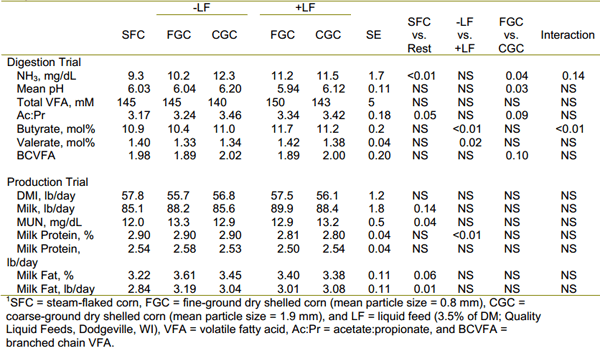
We just analyzed another trial with liquid feeds replacing corn that was either finely ground or coarsely ground and compared those data to a control with steamflaked corn (Table 2). In this trial, though, NFC was maintained at about 36% in diets with forage NDF maintained between 20 and 21%. Cows fed the steam-flaked corn diets had the lowest milk fat yield. Despite the higher butyrate and valerate molar percentages for liquid feed diets, there apparently was not enough difference in particle size of ground corn for liquid feeds to interact for milk fat yield. Decreasing rumen degradable starch in the previous trial (Table 1) was associated with an increase in DMI and milk fat yield. In the current trial, grinding corn more finely increased the soluble dry matter concentration but did not affect the rate of DM degradation of the potentially degradable pool (data not shown), and NDF digestibilities (51.8 to 56.8%) were not affected by treatment. Thus, these data support previous discussions that sugars do not increase the likelihood for milk fat depression, but our expectation to improve milk fat more with addition of liquid feed to coarsely ground corn than to finely ground corn (a statistical interaction) was not realized. Perhaps the lack of change in DMI was associated with different dietary conditions in which DMI was not limited by a metabolic response or else non-nutritional benefits of liquid feeds were not realized under the conditions of the current trial.
Conclusions
Sugars at 2.5 to 5% offer potential benefits that should be considered when formulating dairy rations. Based on current information, I recommend keeping NFC inclusion at < 37% in corn silage-based diets but perhaps < 40% in alfalfa-based diets; starch inclusion should be < 25% for corn silage diets, but perhaps can be higher with alfalfa or grass diets. When NFC and especially rumen-degraded starch are kept at these moderate concentrations, sugars are more likely to stimulate DMI, NDF digestibility, and milk fat production. Adding sugars to diets with Rumensin does not increase the risk of milk fat depression. Moreover, particularly when in the form of liquid feeds applied to the TMR, sugars should help reduce sorting both against forage and for the fines. Ruminal and post-ruminal effects of sugar addition plus feedbunk management for group-fed cows apparently can aggregate for an overall potential benefit in milk production and efficiency.
References
Allen, M. S., B. J. Bradford, and M. Oba. 2009. Board invited review: The hepatic oxidation theory of the control of feed intake and its application to ruminants. J. Anim. Sci. 87:3317-3334.
Arroquy, J. I., R. C. Cochran, M. Villarreal, T. A. Wickersham, D. A. Llewellyn, E. C. Titgemeyer, T. G. Nagaraja, D. E. Johnson, and D. Gnad. 2004a. Effect of level of rumen degradable protein and type of supplemental non-fiber carbohydrate on intake and digestion of low-quality grass hay by beef cattle. Anim. Feed Sci. Technol. 115:83-99.
Arroquy, J. I., R. C. Cochran, T. A. Wickersham, D. A. Llewellyn, E. C. Titgemeyer, T. G. Nagaraja, and D. E. Johnson. 2004b. Effects of type of supplemental carbohydrate and source of supplemental rumen degradable protein on low quality forage utilization by beef steers. Anim. Feed Sci. Technol. 115:247-263.
Beauchemin, K. A., D. Colombatto, D. P. Morgavi, and W. Z. Yang. 2003. Use of exogenous fibrolytic enzymes to improve feed utililization by ruminants. J. Anim. Sci. 81(E. Suppl. 2):E37-E47.
Broderick, G. A., N. D. Luchini, S. M. Reynal, G. A. Varga, and V. A. Ishler. 2008. Effect on production of replacing dietary starch with sucrose in lactating dairy cows. J. Dairy Sci. 91:4801-4810.
Broderick, G. A., and W. J. Radloff. 2004. Effect of molasses supplementation on the production of lactating dairy cows fed diets based on alfalfa and corn silage. J. Dairy Sci. 87:2997-3009.
Calsamiglia, S., M. Busquet, P. W. Cordozo, L. Castillejos, and A. Ferret. 2007. Invited Review: Essential oils as modifiers of rumen microbial fermentation. J. Dairy Sci. 90:2580-2595.
Calsamiglia, S., P. W. Cardozo, A. Ferret, and A. Bach. 2008. Changes in rumen microbial fermentation are due to a combined effect of type of diet and pH. J. Anim. Sci. 86:702-711.
Charbonneau, E., P. Y. Chouinard, G. Allard, H. Lapierre, and D. Pellerin. 2006. Milk from forage as affected by carbohydrate source and degradability with alfalfa silage-based diets. J. Dairy Sci. 89:283-293.
Colombatto, D., and K. A. Beauchemin. 2009. A protease additive increases fermentation of alfalfa diets by mixed ruminalmicroorganisms in vitro. J. Anim. Sci. 87:1097-1105.
DeFrain, J. M., A. R. Hippen, K. F. Kalscheur, and D. J. Schingoethe. 2006. Feeding lactose to increase ruminal butyrate and the metabolic status of transition dairy cows. J. Dairy Sci. 89:267-276.
Edwards, J. E., S. A. Huws, E. J Kim, and A. H. Kingston-Smith. 2007. Characterization of the dynamics of initial bacterial colonization of nonconserved forage in the bovine rumen. FEMS Microbiol. Ecol. 62:323-335.
Emmanuel, D. G. V., K. L. Madsen, T. A. Churchill, S. M. Dunn, and B. N. Ametaj. 2007. Acidosis and lipopolysaccharide from Escherichia coli B:055 cause hyperpermeability of rumen and colon tissues. J. Dairy Sci. 90:5552-5557.
Firkins, J. L., M. L. Eastridge, N. R. St-Pierre, and S. M. Noftsger. 2001. Effects of grain variability and processing on starch utilization by lactating dairy cattle. J. Anim. Sci. 79(E. Suppl.):E218-E238.
Firkins, J. L., A. N. Hristov, M. B. Hall, G. A. Varga, and N. R. St-Pierre. 2006. Integration of ruminal metabolism in dairy cattle. J. Dairy Sci. 89(E. Suppl.):E31- E51
Firkins, J. L., S. K. R. Karnati, and Z. Yu. 2008a. Linking rumen function to animal response by application of metagenomics techniques. Aust. J. Exp. Agric. 48:711-721.
Firkins, J. L., B. S. Oldick, J. Pantoja, L. E. Gilligan, and L. Carver. 2008b. Efficacy of liquid feeds varying in concentration and composition of fat, non-protein nitrogen, and non-fiber carbohydrates for lactating dairy cows. J. Dairy Sci. 91:1969-1984.
Fron, M., H. Madeira, C. Richards, and M. Morrison. 1996. The impact of feeding condensed distillers byproducts on rumen microbiology and metabolism. Anim. Feed Sci. Technol. 61:235-245.
Fuentes, M. C., S. Calsamiglia, P. W. Cardozo, and B. Vlaemink. 2009. Effect of pH and level of concentrate in the diet on the production of biohydrogenation intermediates in a dual-flow conntinuous culture. J. Dairy Sci. 92:4456-4466.
Gozho, G. N., D. O. Krause, and J. C. Plaizier. 2007. Ruminal lipopolysaccharide concentration and inflammatory response during grain-induced subacute ruminal acidosis in dairy cows. J. Dairy Sci. 90:856-866.
Griswold, K. E., G. A. Apgar, J. Bouton, and J. L. Firkins. 2003. Effects of urea infusion and ruminal degradable protein concentration on microbial growth, digestibility, and fermentation in continuous culture. J. Anim. Sci. 81:329-336.
Guan, L. L., J. D. Nkrumah, J. A. Basarab, and S. S. Moore. 2008. Linkage of microbial ecology to phenotype: correlation of rumen microbial ecology to cattle´s feed efficiency. FEMS Microbiol. Lett. 288:85-91.
Heldt, J. S., R. C. Cochran, G. L. Stokka, C. G. Farmer, C. P. Mathis, E. C. Titgemeyer, and T. G. Nagaraja. 1999. Effects of different supplemental sugars and starch fed in combination with degradable intake protein on low-quality forage use by beef steers. J. Anim. Sci. 77:2793-2802.
Henning, P. H, C. H. Horn, K.-J. Leeuw, H. H. Meissner, and F. M. Hagg. 2010. Effect of ruminal administration of the lactate-utilizing strain Megasphaera elsdenii (Me) NCIMB 41125 on abrupt or gradual transition from forage to concentrate diets. Anim. Feed Sci. Technol. 157:20-29.
Iqbal, S., Q. Zebeli, A. Mazzolari, G. Bertoni, S. M. Dunn, W. Z. Yang, and B. N. Ametaj. 2009. Feeding barley grain steeped in lactic acid modulates rumen fermentation patterns and increases milk fat content in dairy cows. J. Dairy Sci. 92:6023-6032.
Iqbal, S., Q. Zebeli, A. Mazzolari, S. M. Dunn, and B. N. Ametaj. 2010. Feeding rolled barley grain steeped in lactic acid modulated energy status and innate immunity in dairy cows. J. Dairy Sci. 93:5147-5156.
Jenkins, T. C., R. J. Wallace, P. J. Moate, and E. E. Mosley. 2008. Board-Invited Review: Recent advances in biohydrogenation of unsaturated fatty acids within the rumen microbial ecosystem. J. Anim. Sci. 86:397-412.
Khafipour, E., S. Li, J. C. Plaizier, and D. O. Krause. 2009. Rumen microbiome composition determined using two nutritional models of subacute ruminal acidosis. Appl. Environ. Microbiol. 75:7115-7124.
Khafipour, E., J. C. Plaizier, P. C. Aikman, and D. O. Krause. 2011. Population structure of rumen Escherichia coli associated with subacute ruminal acidosis (SARA) in dairy cattle. J. Dairy Sci. 94:351-360.
Kingerman, C. M., W. Hu, E. E. McDonell, M. C. DerBedrosian, and L. Kung, Jr. 2009. An evaluation of exogenous enzymes with amylolytic activity for dairy cows. J. Dairy Sci. 92:1050-1059.
Klieve, A. V., D. Hennessey, D. Ouwerkerk, R. J. Forster, R. I. Mackie, and G. T. Attwood. 2003. Establishing populations of Megasphaera elsdenii YE34 and Butyrivibrio fibrisolvens YE44 in the rumen of cattle fed high grain diets. J. Appl. Microbiol. 95:621-630.
Kristensen, N. B. 2005. Splanchnic metabolism of volatile fatty acids in the dairy cow. Anim. Sci. 80:3-10.
Lesmosquet, S., G. Raggio, G. E. Lobley, H. Rulquin, J. Guinard-Flament, and H. Lapierre. 2009. Whole-body glucose metabolism and mammary energetic nutrient metabolism in lactating dairy cows receiving digestive infusions of casein and propionic acid. J. Dairy Sci. 92:6068-6082.
Lettat, A., P. Nozière, M. Silberberg, D. P. Morgavi, C. Berger, and C. Martin. 2010. Experimental feed induction of ruminal lactic, propionic, or butyric acidosis in sheep. J. Anim. Sci. 88:3041-3046.
Martin, S. A. 1998. Manipulation of ruminal fermentation with organic acids: A review. J. Anim. Sci. 76:3123-3132.
Martin, S. A., and D. J. Nisbet. 1992. Effect of direct-fed microbials on rumen microbial fermentation. J. Dairy Sci. 75:1736-1744.
Miller-Cushon, E. K., and T. J. DeVries. 2009. Effect of dietary dry matter concentration on the sorting behavior of lactating dairy cows fed a total mixed ration. J. Dairy Sci. 92:3292-3298.
Mouriño, F., R. Akkarawongsa, and P. J. Weimer. 2001. Initial pH as a determinant of cellulose digestion rate by mixed ruminal microorganisms in vitro. J. Dairy Sci. 84:848-859.
Murphy, M. R., A. W. P. Geijsel, E. C. Hall, and R. D. Shanks. 1997. Dietary variety via sweetening and voluntary feed intake by lactating dairy cows. J. Dairy Sci. 80:894-897.
Nagaraja, T. G., and E. C. Titgemeyer. 2007. Ruminal acidosis in beef cattle: The current microbiological and nutritional outlook. J. Dairy Sci. 90(E. Suppl.):E17- E38.
Nousiainen, J., M. Rinne, and P. Huhtanen. 2009. A meta-analysis of feed digestion in dairy cows. 1. The effects of forage and concentrate factors on total diet digestibility. J. Dairy Sci. 92:5019-5030.
Oelker, E. R., C. Reveneau, and J. L. Firkins. 2009. Interaction of molasses and monensin in alfalfa hay- or corn silage-based diets on rumen fermentation, total tract digestibility, and milk production by Holstein cows. J. Dairy Sci. 92:270-285.
Oldick, B. S., J. L. Firkins, and N. R. St-Pierre. 1999. Estimation of microbial nitrogen flow to the duodenum of cattle based on dry matter intake and diet composition. J. Dairy Sci. 82:1497-1511.
Palmonari, A., D. M. Stevenson, D. R. Mertens, C. W. Cruywagen, and P. J. Weimer. 2010. pH dynamics and bacterial community composition in the rumen of lactating dairy cows. J. Dairy Sci. 93:279-287.
Palmquist, D. L., C. L. Davis, R. E. Brown, and D. S. Sachan. 1969. Availability and metabolism of various substrates in ruminants. V. Entry rate into the body and incorporation into milk fat of D(-)b-hydroxybutyrate. J. Dairy Sci.-633.
Penner, G. B., J. R. Aschenbach, G. Gäbel, R. Rackwitz, and M. Oba. 2009. Epithelial capacity for apical uptake of short chain fatty acids is a key determinant for intraruminal pH and the susceptibility to subacute ruminal acidosis in sheep. J. Nutr. 139:1714-1720.
Penner, G. B., and M. Oba. 2009. Increasing dietary sugar concentration may improve dry matter intake, ruminal fermentation, and productivity of dairy cows in the postpartum phase of the transition period. J. Dairy Sci. 92:3341-3353.
Piwonka, E. J., and J. L. Firkins. 1996. Effect of glucose fermentation on fiber digestion by ruminal microorganisms in vitro. J. Dairy Sci. 79:2196-2206.
Ribeiro, C. V. D. M., S. K. R. Karnati, and M. L. Eastridge. 2005. Biohydrogenation of fatty acids and digestibility of fresh alfalfa or alfalfa hay plus sucrose in continuous culture. J. Dairy Sci. 88:4007-4017.
Rychlik, J. L., R. LaVera, and J. B. Russell. 2002. Amino acid deamination by ruminal Megasphaera elsdenii strains. Curr. Microbiol. 45:340-345.
Rychlik, J. L., and J. B. Russell. 2000. Mathematical estimations of hyper-ammonia producing ruminal bacteria and evidence for bacterial antagonism that decreases ruminal ammonia production. FEMS Microbiol. Ecol. 32:121-128.
Storm, A. C., and N. B. Kristensen. 2010. Effects of forage particle size and dry matter content of a total mixed ration on intraruminal equilibration and net portal flux of volatile fatty acids in lactating dairy cows. J. Dairy Sci. 93:4223-4238.
Walker, N. D., C. J. Newbold, and R. J. Wallace. 2005. Nitrogen metabolism in the rumen. Pages 71-115 in Nitrogen and Phosphorus Nutrition of Cattle. E. Pfeffer and A. Hristov, eds. CABI Publishing, Cambridge, MA.
Wallace, R. J. 2008. Gut microbiology - broad genetic diversity, yet specific metabolic niches. Animal 2:661-668.
Weimer, P. J., D. M. Stevenson, and D. R. Mertens. 2010. Shifts in bacterial community composition in the rumen of lactating dairy cows under milk fat-depressing conditions. J. Dairy Sci. 93:265-278.
Weiss, W. P., N. R. St-Pierre, and L. B. Willett. 2009. Varying type of forage, concentration of metabolizable protein, and source of carbohydrate affects nutrient digestibility and production by dairy cows. J. Dairy Sci. 92:5595-5606.
Welkie, D. G., D. M. Stevenson, and P. J. Weimer. 2010. ARISA analysis of ruminal bacterial community dynamics in lactating dairy cows during a feeding cycle. Anaerobe 16:94-100.
Wu, Z., and J. T. Huber. 1994. Relationship between dietary fat supplementation and milk protein concentration in lactating cows: A review. Livest. Prod. Sci. 39:141- 155.
Zebeli, Q., and B. N. Ametaj. 2009. Relationships between rumen lipopolysaccharide and mediators of inflammatory response with milk fat production and efficiency in dairy cows. J. Dairy Sci. 92:3800-3809.
Zebeli, Q., B. N. Ametaj, B. Junck, and W. Drochner. 2009. Maize silage particle length modulates feeding patterns and milk composition in loose-housed lactating Holstein cows. Livest. Sci. 124:33-40.
Zebeli, Q., D. Mansmann, H. Steingass, and B. N. Ametaj. 2010. Balancing diets for physically effective fibre and ruminally degradable starch: A key to lower the risk of sub-acute rumen acidosis and improve productivity of dairy cattle. Livest. Sci. 127:1-10.
Table 1. Lactation performance by dairy cattle fed diets containing different concentrations of nonstructural carbohydrates without or with Rumensin ®.
Table 2. Fermentation and lactation performance by cows fed different sources of grain without or with liquid feed.
Figure 1. Chopping length of kernel-processed corn silage influences the eating behavior of dairy cows fed TMR containing 40% corn silage, 10% grass hay, and 50% concentrate. Top panel: the number of meals (n) was quadratically related to particle length during the day (A.M., P < 0.05) and cumulative responses (A.M. + P.M., P < 0.08). Bottom panel: the as fed amount of feed consumed per meal was linearly (P < 0.05) and quadratically (P < 0.08) affected by particle length in the evening (P.M.) and linearly (P < 0.09) affected when the A.M. plus P.M. meals were averaged (not shown). Data are from Zebeli et al. (2009).
This paper was presented at the 22nd Annual Florida Ruminant Nutrition Symposium at the Best Western Gateway Grand Hotel, Gainesville, Florida, USA. February 1-2, 2011. Engormix.com thanks the author and the organizing committee for this huge contribution.