Factors Affecting Fish Blood Profile: B- Effect of Environmental and Genetic Factors
Published: March 1, 2021
By: Abdelhamid M. Abdelhamid 1; Mohamed M. A. H. Refaey 1; Mahmoud F. Salem 2 and Mostafa A. M. El-Kattan 1. / 1 Animal Production Department, Faculty of Agriculture, Mansoura University, Egypt; 2 Aquaculture Research Unit, Sakha, Central Lab. of Aquaculture Research, Agricultural Research Center, Cairo, Egypt.
Summary
Continued studies were undertaken to complete understanding various factors affecting blood composition of fish. In this part (B) of the present serial studies, we gave light on effect of some environmental and genetic factors, mainly water temperature, narcosis, handling stress, poly-culture, farm conditions, starvation, fish species, sex, and size. All these factors had affected most studied parameters of the haematology /and or the biochemistry of fish blood.
Introduction
Hematological parameters of fish blood are useful tools that aid in the study immuno-potentiators. Such tests are general but not conclusive and must be correlated with biochemical tests of the subject. The classification of leucocytes as in the vertebrates has been done following morphological criteria where by various groups can be distinguished such as lymphocytes, granulocytes and macrophages (Ellis, 1981). Dominguez et al. (2004) confirmed that elevating rearing water temperature for 2 weeks increased the circulating IgM concentration in Nile tilapia, but rearing the fish at 33 ºC resulted in a decrease in IgM concentration, suggesting that the fish possess an appropriate thermal range for production of immune substances. Plasma level of IgM increased significantly with salinity at 12 and 24 ppt. On the other hand, plasma IgM level did not change by exposing the fish to acidification and suspended solids. These results suggest that the specific immune system of tilapia changes by certain factors in aquatic environment. Adam (2007) registered a negative correlation between hematological indices and water quality parameters with moderate regression factors. Relationship between RBC and water quality characteristics was positive and with very low regression factor. However, the present study was designed to evaluate the effect of some factors affecting some blood parameters. These factors included fish species, sex and age; water salinity and pollution; rearing conditions; dietary treatments; since it was given in Verse 12 of Sura Faatir of Holly Quran concerning the variations in water and fish composition: "And not alike are the two bodies of water. One is fresh and sweet, palatable for drinking, and one is salty and bitter. And from each you eat tender meat and extract ornaments which you wear, and you see the ships plowing through [them] that you might seek of His bounty; and perhaps you will be grateful". So, this was also included in the objectives of the present study.
Materials and methods
Random fish samples were taken for blood collected from the caudal peduncle by special syringe, adequate amount of whole blood was withdrawn in small plastic vials containing EDTA (ethylene diamine tetra acetic acid) as anticoagulant and used to obtain the blood plasma by centrifuge at 3500 rpm for 15 min. Blood plasma samples were used for determination of creatinine (Tietz, 1986), triglycerides (McGowan et al., 1983), total proteins (Tietz, 1990) and albumin (Wotton and Freeman, 1982) concentrations as well as the activity of aspartate amino transferase (AST) and alanine amino transferase (ALT) using commercial test kits in a private lab. in Kafr El-Sheikh governorate, Egypt. Globulin level was calculated by subtracting albumin from total protein. The other samples of blood were used to determine the blood hematology as concentration of hemoglobin (Hb), total count of erythrocytes (RBCs), and total leukocytes (WBCS) (Natt and Herrick, 1952) and hematocrit (Hct) using Auto Counter (Decie and Lewis, 2006) in the same lab. The other hematological parameters were mathematically calculated.
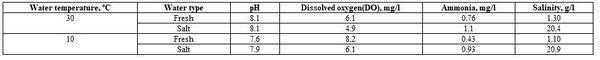
- To study the effects of water temperature and fish species and size, five fishes from each body weight (50±3, 150±3, and 250±3 g) and each freshwater species (Nile tilapia, African catfish, and striped mullet) were collected from a fish farm in Tolompat-7- Alreiad, Kafr El-Sheikh governorate. Concerning saltwater fish, five fishes from each body weight (50±3, 150±3, and 250±3 g) and fish species (sea bream and sea bass) were gathered from a fish farm in Mosalas Al-Diba, Damietta governorate. The fish samples were collected during both summer (September, at water temperature 30ºC) and winter (January, at water temperature 10 ºC) seasons. The water quality conditions were as follow:
- To study the effect of some narcotic agents, before and after the anesthesia, on male and female of Nile tilapia and African catfish on the blood profile, three agents (MS222, Qunaldin, and clove oil) were used. The levels used for narcosis were 75 mg/l MS222 for 20 min., 9μg/l ethyl alcohol Qunaldin for 15 min., and 1-3 ml clove oil/10 l alcohol added to 10 l water for 20 min. This trial was conducted during March 2019. The rearing water conditions were 20.7ºC, 7.5 pH, 4.9 mg/l DO, 0.61 mg/l ammonia, and 1.2 g/l salinity. Five fishes from each sex within each fish species were taken from a fish farm in the district of Ard Al-Shorta, Baltim, Kafr Al-Sheikh governorate. The fish body weights were 400±3 g for both males and females Nile tilapia and 250±3 and 300±3 g for males and females African catfish, respectively.
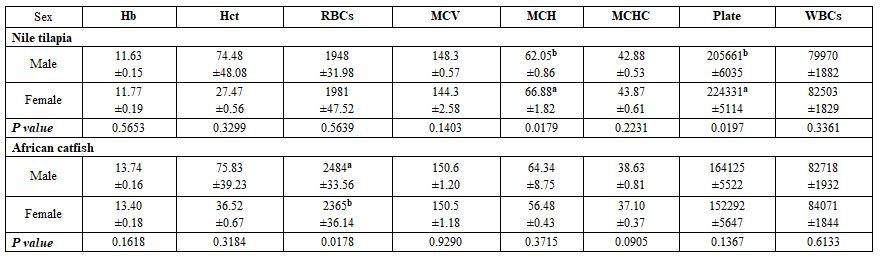
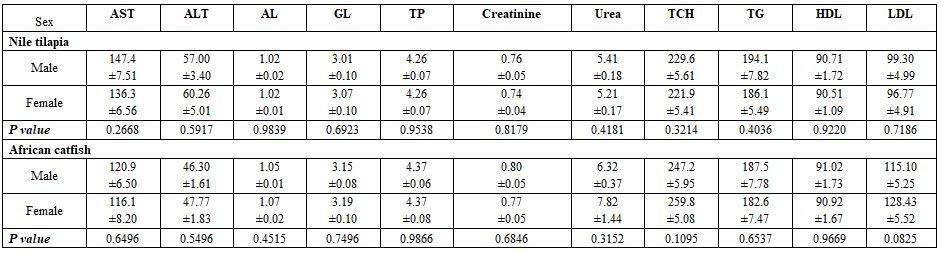
- To study the effect of fish species and sex under poly-culture system on the blood picture, five fishes from each sex (male and female) within each species (Nile tilapia, African catfish, striped mullet, and common carp) from a fish farm in Ma'zoor district, Al-Hamol, Kafr Al-Sheikh governorate working with the poly-culture system. The earthen pond in this farm had an area of 40 Fadden, stocked with million tilapia fry, 20 thousand striped mullet, 10 thousand common carp, and 5 thousand African catfish. The samples' fish weights were 300±3 and 250±3 g for males and females Nile tilapia, 850±3 and 300±3 g for males and females African catfish, 240±3 and 270±3 g for males and females striped mullet, and 850±3 g for either males and females common carp, respectively. The fish rearing water has 21ºC, 8.3 pH, 5.1 mg/l DO, 0.74 mg/l ammonia, and 1.6 g/l salinity.
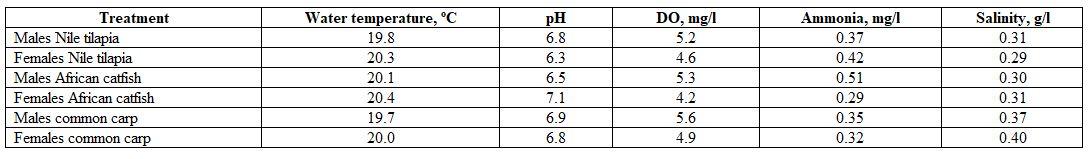
- For studying the effect of fish species and sex on the haematology and biochemistry under handling stress, during March 2019, five males and five females from Nile tilapia (400±3 g for each sex), African catfish (200±3 and 300±3 g, respectively), and common carp (1000±3 g for both sexes). The rearing water conditions were 20.3ºC, 6.1 pH, 4.5 mg/l DO, 0.32 mg/l ammonia, and 1.4 g/l salinity. Fish samples were taken from a fish farm in the district of Ard Al-Shorta, Baltim, Kafr Al-Sheikh governorate, and then transferred into glass aquaria for 24 h. About six Nile tilapia and African catfish were stocked into each glass aquarium with 200-210 l water, whereas common carp were stocked into glass aquaria individually. Each sex was given in a separate aquarium. After 24 h., blood samples were withdrawing, and fish were returned to aquaria for one hour. Thereafter, fish were net handled out- and in-water 5-6 times, each for 15 sec. and repeated for 20 min., then another blood samples were withdrawing. The water quality criteria in this experiment were as follow:
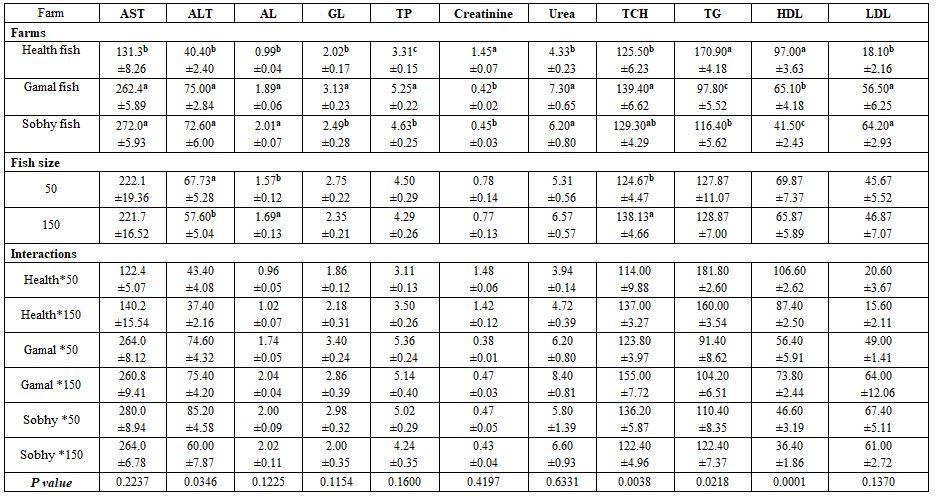
- To study the effect of farm location on blood profile of different sizes of Nile tilapia, five fishes from either sizes (150±3 and 250±3 g) from three different farms concerning the environmental conditions, were collected during August 2017. The 1st farm in Baltim, Kafr El-Sheikh governorate, its fishes were normal without mortality and apparently sound. Its water had 30.2 ºC, 7.5 pH, 6.1 mg/l DO, 0.36 mg/l ammonia, and 1.1 g/l salinity. The 2nd farm located at Tolombat-7, Al-Riad (Fathy El-Gammal), Kafr El-Sheikh governorate, it had 100 died fish daily, with external symptoms (protruded eyes). Its water had 31.7 ºC, 4.7 pH, 3.2 mg/l DO, 2.9 mg/l ammonia, and 1.1 salinity. The 3rd farm located at Siag-Hamol (Sobhy Halawa), Kafr El-Sheikh governorate, it had more death cases than the former, ca. 300 fish daily, with symptoms: protruded eyes, large red-orange spots on the hind part, the post-mortem examination showed enlarged spleen, intestinal edema, pale-enlarged liver, its water had 29.3 ºC, 9.2 pH, 4.3 mg/l DO, 1.2 mg/l ammonia, and 2.1 g/l salinity.
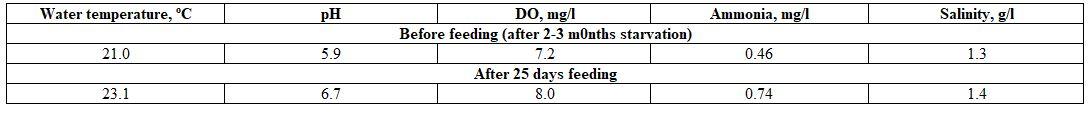
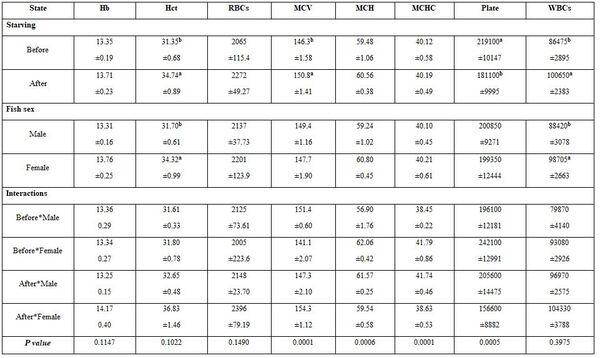
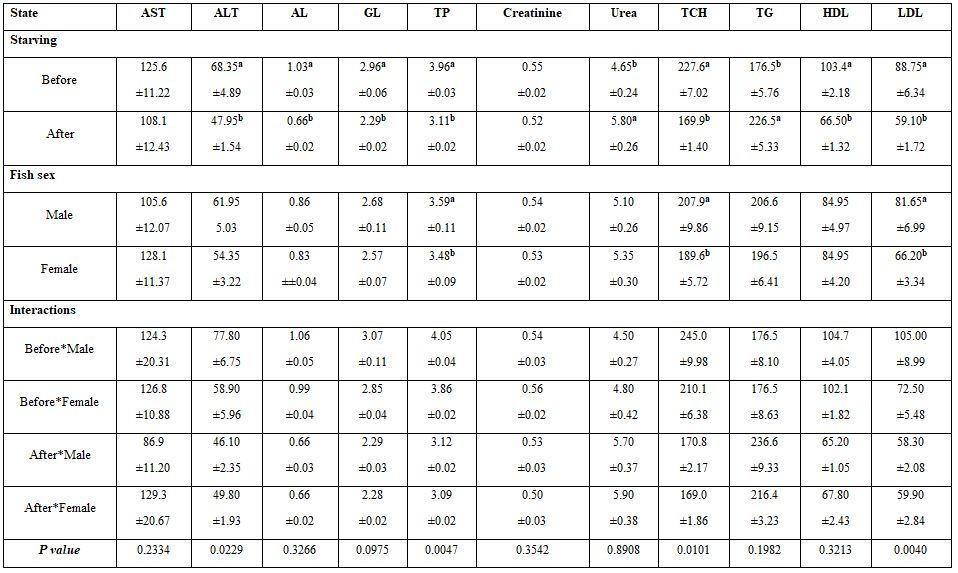
- To study the effect of starvation on the blood profile of males and females Nile tilapia, blood samples were withdrawn during March 2019 the district-77, Al-Hamol, Kafr El-Sheikh governorate, from five fishes from each sex (male and female) and size (100±3 and 150±3g for either sexes) after 2-3 months of starvation. The farm fish were re-fed for 25 days with Aqua-sinking feed 30% crude protein; thereafter, another blood samples were gathered. Water quality criteria before and after fish feeding were:
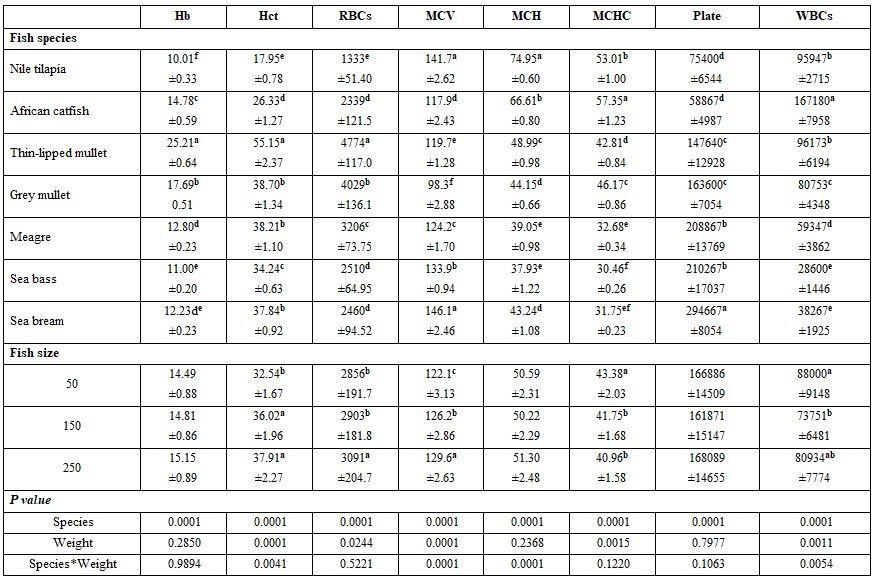
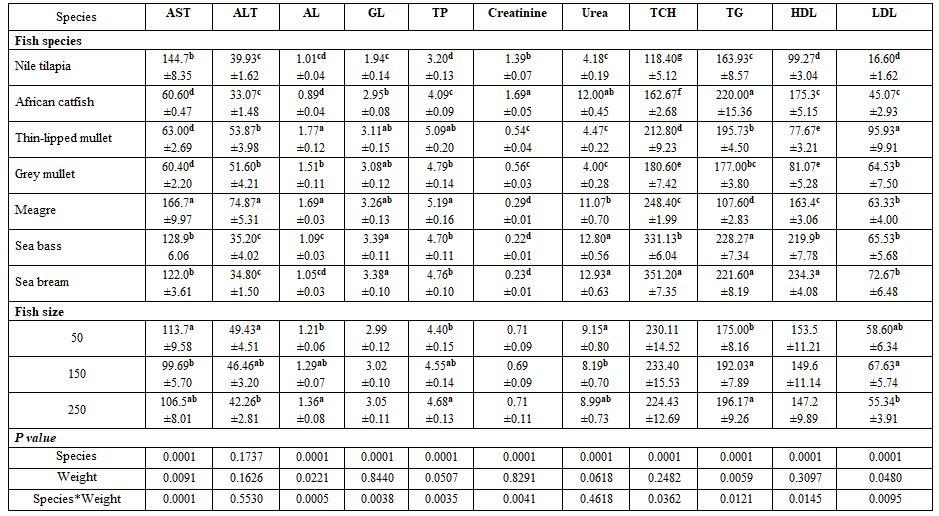
- To evaluate blood picture of fish as affected by species and size of different fresh and salt water fishes, five fishes were collected from each fish species [Nile tilapia, African catfish, grey mullet, and thin-lipped mullet (from a fish farm at Tolombat-7, Al-Reiad, Kafr El-Sheikh governorate during August 2017, where its water had 30.1 ºC, 8.1 pH, 5.3 mg/l DO, 0.45 mg/l ammonia, and 1.3 g/l salinity) as well as sea bream, sea bass (from a fish farm in Mosalas Aldiba, Damietta governorate during October 2017, where its water had 27 ºC, 7.5 pH, 6.7 mg/l DO, 0.78 mg/l ammonia, and 22.1 g/l salinity), and meager (from a fish farm at Bar-Bahary, Baltim, Kafr El-Sheikh governorate during August 2017, where its water had 31.3 ºC, 5.8 pH, 3.4 mg/l DO, 0.46 mg/l ammonia, and 12.9 g/l salinity)] and size (50±3, 150±3, and 250±3 g).
- To compare between both sexes for Nile tilapia and African catfish from different locations concerning blood parameters, five fishes (250±3 g for each sex, species, and location) from each of males and females of Nile tilapia and African catfish from three different locations (River Nile, Lake Borulus, and a fish farm) during September 2018. The water quality in these locations were:
Blood analysis data was statistically analyzed according to SAS (2006). When F- test was significant at P≤0.05, Duncan's test (1955) was carried out.
Results
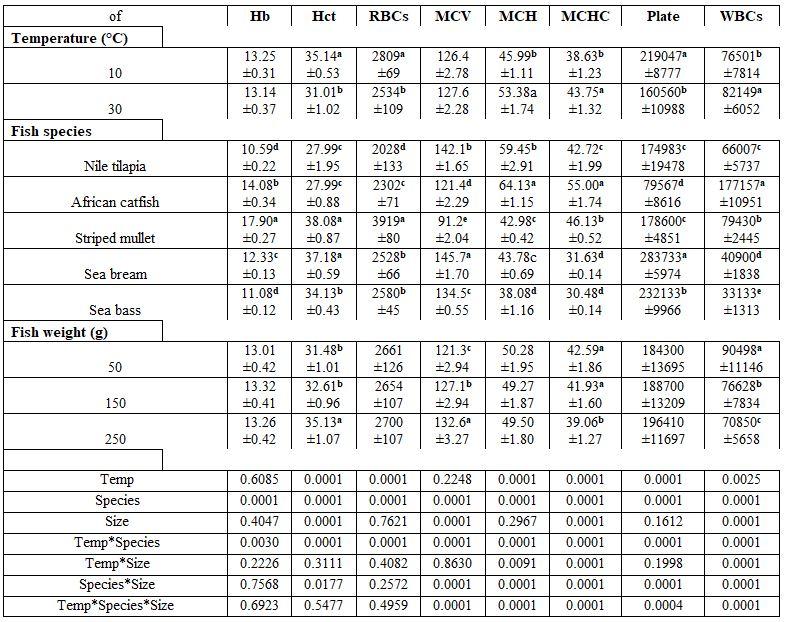
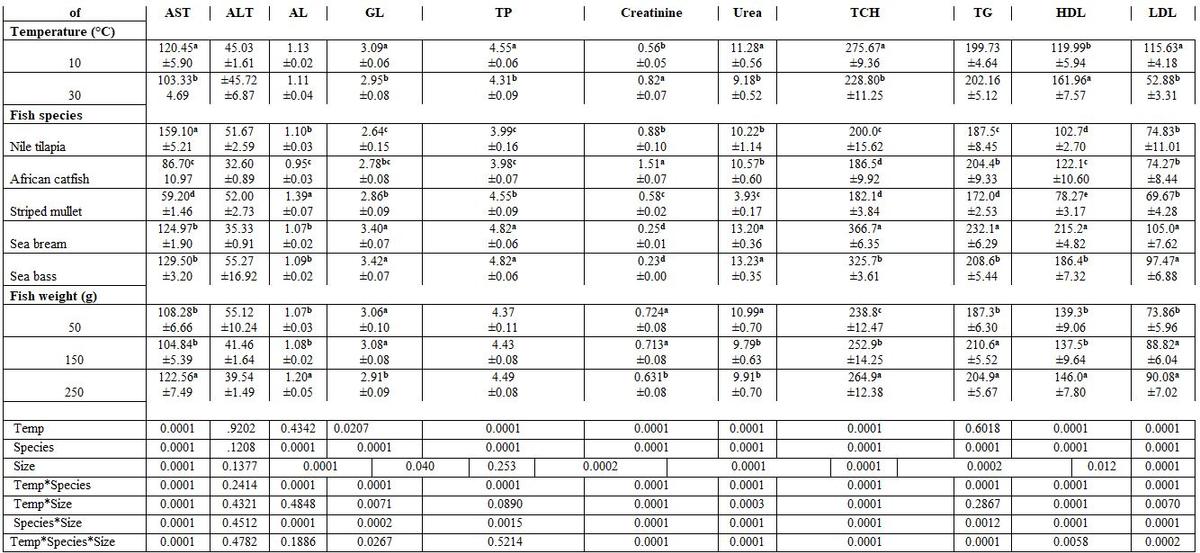
- Water temperature and fish species and size: Fish rearing water temperature significantly affected all haematological parameters tested, except haemoglobin (Hb) and MCV; whereas, fish species significantly affected all of them. But fish weight significantly affected each of Hct, MCV, MCHC, and WBCs. Most interactions were significant for MCV, MCH, MCHC, platelets, and WBCs (Table 1). Elevating water temperature reduced all values of the biochemical parameters test. Except alanine aminotransferase (ALT), all other biochemical parameters were significantly affected by fish species and weight. The interactions among these variables were also significant, except for ALT, albumin (AL), total protein (TP), and TG (Table 2).
Table 1: On haematological parameters
a-e: means superscripted with different letters in the same column and group differ significantly at P≤0.05.
Table 2: On biochemical parameters
a-e: means superscripted with different letters in the same column and group differ significantly at P≤0.05.
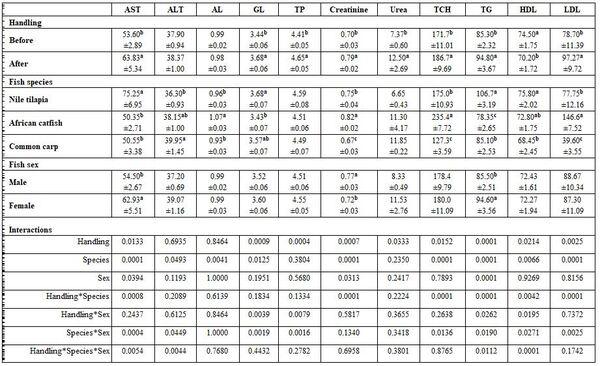
- Anesthesia type, before and after anesthesia, and fish species and sex: Table 3 shows different dimensions for the effect of different narcotic substances, before and after anesthesia, and fish species and sex as well as their interactions concerning the haematological measurements. It is worth noting that MS222 elevated Hb, Hct, RBCs, and WBCs values comparing with the other two narcotic agents. Yet, clove oil particularly reduced Hct, RBCs, and platelets. But Quinaldin mainly reduced Hb and WBCs. MS222 elevated ALT and urea values while clove oil increased the level of AL, TCH, and LDL comparing with the other agents. Fish species and sex significantly affected only the activity of both enzymes AST and ALT. Significant interactions were calculated too (Table4).
Table 3: On haematological parameters
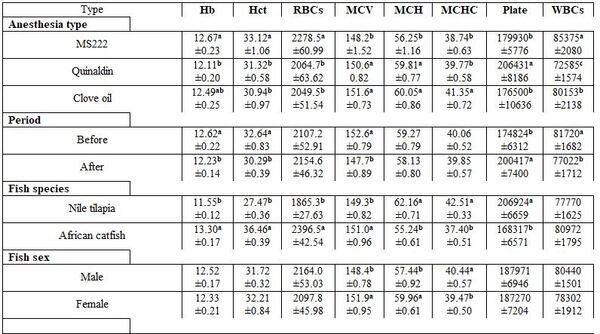
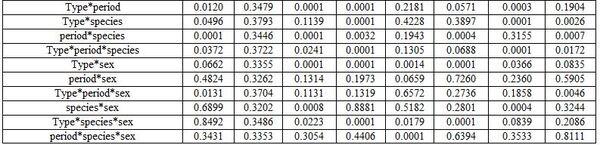
a-c: means superscripted with different letters in the same column and group differ significantly at P≤0.05.
Table 4: On biochemical parameters
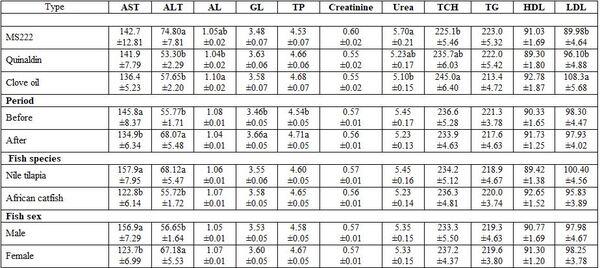
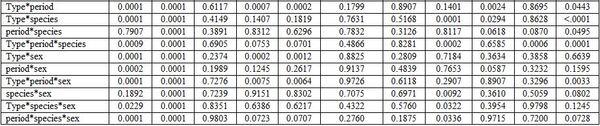
a-b: means superscripted with different letters in the same column and group differ significantly at P≤0.05.
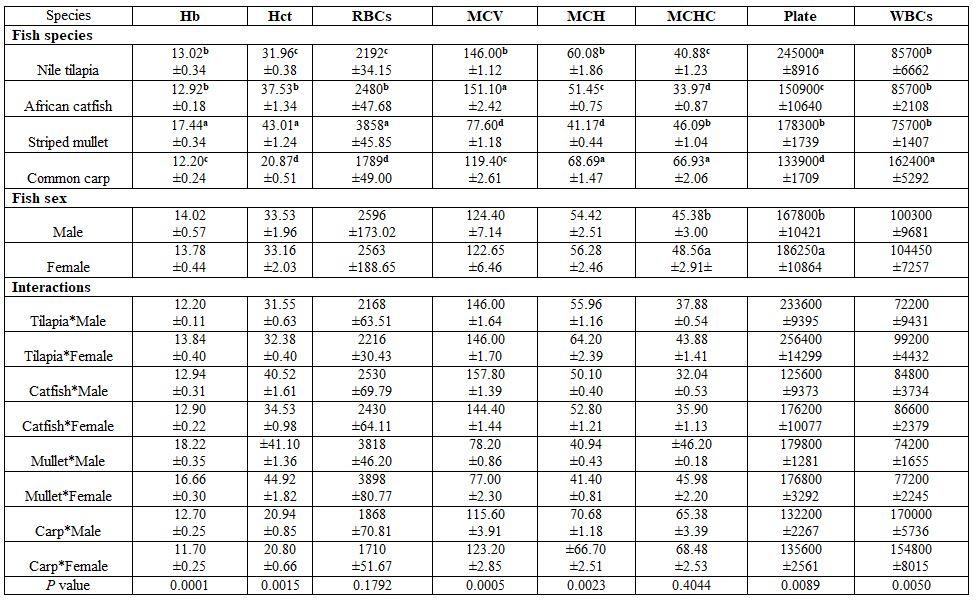
Fish species and sex under poly-culture system: All haematological and biochemical parameters were significantly affected by fish species; yet, fish sex significantly affected only MCHC, TG, and LDL values (Tables 5 and 6). Most interactions were significant for the haematology but not for the biochemical parameters.
Table 5: On haematological parameters
a-d: means superscripted with different letters in the same column and group differ significantly at P≤0.05.
Table 6: On biochemical parameters
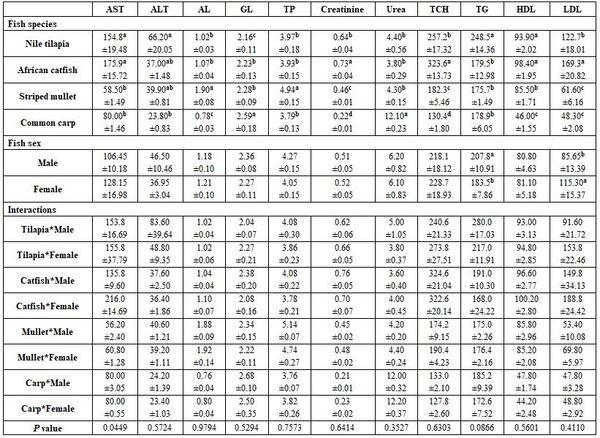
a-d: means superscripted with different letters in the same column and group differ significantly at P≤0.05.
Fish species and sex under handling stress: Handling stress significantly affected all haematological and biochemical parameters, except Hct, WBCs, ALT, and AL, but fish species did not significantly affect MCH, TP, and urea. Fish sex affected only Hb, platelets, and WBCs, AST, creatinine, and TG (Tables 7 and 8).
Table 7: On haematological parameters
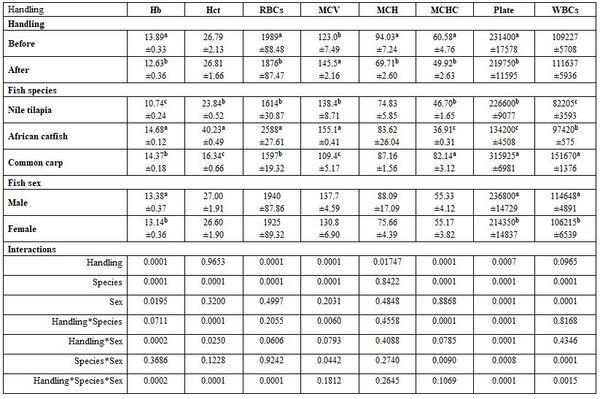
a-c: means superscripted with different letters in the same column and group differ significantly at P≤0.05.
Table 8: On biochemical parameters
a-c: means superscripted with different letters in the same column and group differ significantly at P≤0.05.
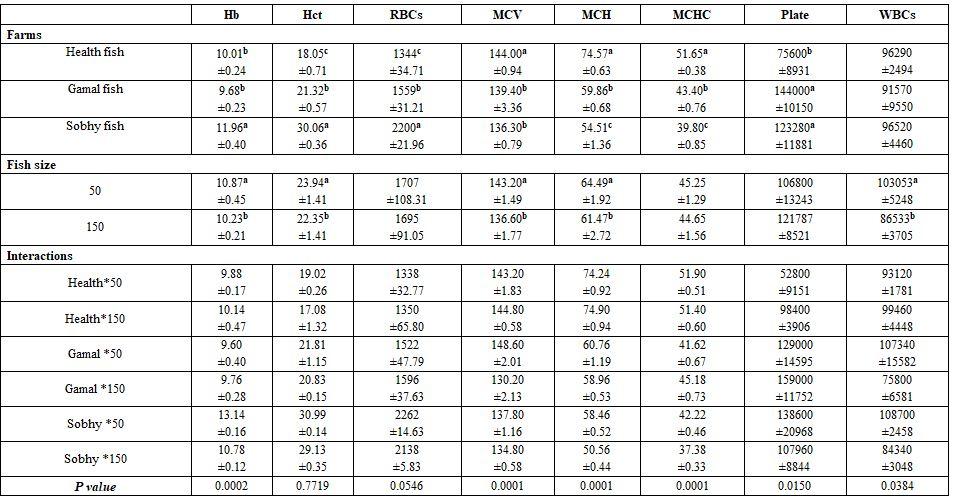
Fish farm location and fish size: Fish farm conditions affected significantly but unexpected that the best-sound farm (1st) fishes reflected lower Hb, Hct, RBCs, ASt, ALT, AL, GL, TP, urea, and TCH (Tables 9 and 10) than the worst farm's fish (3rd, Sobhy) which reflected high mortality with pathogenic symptoms, as given in the materials. The big sized fish gave significantly lower haematological values than the smaller ones. The interactions were more significant for most haematological than the biochemical parameters.
Table 9: On haematological parameters
a-c: means superscripted with different letters in the same column and group differ significantly at P≤0.05.
Table 10: On biochemical parameters
a-c: means superscripted with different letters in the same column and group differ significantly at P≤0.05.
- Starvation and fish sex: Starvation significantly increased each of Hct, MCV, WBCs, urea, and TG (Tables 11 and 12). Males had lower significantly Hct and WBCs, but higher TP, TCH, and LDL values. The interactions were more significant for most haematological than the biochemical parameters.
Table 11: On haematological parameters
a-b: means superscripted with different letters in the same column and group differ significantly at P≤0.05.
Table 12: On biochemical parameters
a-b: means superscripted with different letters in the same column and group differ significantly at P≤0.05.
7) Fish size and species (salt-and freshwater fishes): Highest Hb, Hct, RBCs, Al, and LDL were reported for thin-lipped mullet (Tobara). Freshwater fishes had higher WBCs counts and creatinine concentration but lower GL, TCH than the saltwater fishes Tables 13 and 14). Fish size in the interactions had also significant effects.
Table 13: On haematological parameters
a-f: means superscripted with different letters in the same column and group differ significantly at P≤0.05.
Table 14: On biochemical parameters
a-g: means superscripted with different letters in the same column and group differ significantly at P≤0.05.
8) Fish sex in Nile tilapia and African catfish: Fish sex significantly affected only MCH and platelets in Nile tilapia and RBCs in African catfish (Tables 15 and 16).
Table 15: On haematological parameters
a-b: means superscripted with different letters in the same column and group differ significantly at P≤0.05.
Table 16: On biochemical parameters
Discussion
Hassanen et al. (1997) working on sea bass (Dicentrarchus labrax), found that neither trash fish nor pelleted feed had significant effect on hematocrit value, hemoglobin concentration, and mean corpuscular hemoglobin. Yet, serum total protein content decreased significantly as the percentage pelleted feed increased. Daghash and Hussein (1999) found that injecting African catfish, Clarias gariepinus with GnRH resulted in increasing the serum glucose and total cholesterol for male and female fish while serum total protein elevated only in females. Heat stress significantly increased hemoglobin and hematocrit values as well as serum glucose concentration, and serum activity of AST and ALT; but it lowered the serum activity of alkaline phosphatase and serum total protein concentration for the decrease in serum globulin rather than albumin in Nile tilapia. These negative effects of heat stress could be counteracted by dietary supplementation of ascorbic acid (Hussein and Kobeisy, 1999). Moreover, Dominguez et al. (2004) confirmed that elevating rearing water temperature (18.4, 23, and 28 ºC) for 2 weeks increased the circulating IgM concentration in Nile tilapia, but rearing the fish at 33 ºC resulted in a decrease in IgM concentration, suggesting that the fish possess an appropriate thermal range for production of immune substances. Plasma level of IgM increased significantly with salinity at 12 and 24 ppt. On the other hand, plasma IgM level did not change by exposing the fish to acidification (pH 4.0) and suspended solids (20, 200, and 2000 mg/l). These results suggest that the specific immune system of tilapia changes by certain factors in aquatic environment. Generally, physiological indicators from blood samples may enable heat stress, health, and welfare to be assessed in Siberian sturgeon.
So, Simide et al. (2016) conducted routine blood samples, taken from Siberian sturgeon (Acipenser baerii), to test the robustness of using only physiological indicators to assess heat stress, health, and welfare. Sampling was performed after 1 month of prebiotic dietary supplementation followed by 4 weeks of sublethal heat stress. The expression of heat shock proteins (HSP) was assessed for the first time in sturgeon erythrocytes. HSP70 and HSP90 expression was triggered by both stress and dietary supplementation. Indicators of non-specific immunity were modified mainly by stress. Complement activity increases with stress while lysozyme activity decreases, but to a lesser extent in supplemented fish. The antioxidant capacity increases with stress while oxidant metabolites decrease and overall oxidative stress was lower for fish that received dietary supplementation. The positive impact of dietary supplementation on health status was observable after a stress challenge. Moreover, Gaber (2000) concluded that there was no significant effect of dietary inclusion of graded levels of clove oil (as a growth promoter) on RBCs, Hb, PCV, WBCs, and total serum protein.
Exposure of fish to phenol, copper sulphate, ammonia, hypoxia, acidic water, starvation and cold water resulted in reduction of serum hemolytic activity as compared to the control values. Small (2004) came to the conclusion that isoeugenol sedated channel catfish (Ictalurus punctatus) and lowered plasma cortisol level than the control fish after 15 min. of confinement. No differences in plasma levels of glucose or lactate were observed whether under exposure to high ammonia or sedation with isoeugenol. Fish exposed to high ammonia for 24 h had elevated cortisol levels. Levels of plasma cortisol, glucose and lactate all increased significantly in fish following acute oxygen depletion for 30 min. So, isoeugenol had suppressive effects on channel catfish plasma cortisol levels during confinement and low oxygen conditions, and reduced the plasma lactate response to acute oxygen depletion. Significant elevation in serum complement activity occurred in sea bass fed with alginic acid (Ergosan) and β-glucan (Macrogard) for 15 days. Significant elevation in serum lysozyme, gill and liver head shock protein concentration were observed, whereas a significant increase of complement activity was only observed in fish that received an Ergosan diet for 15 days. On the long-term period (45 days), no significant differences were observed in innate and specific immune parameters (Bagni et al., 2005).
Concentration of glucose significantly increased and concentration of total protein significantly decreased in blood plasma of all groups (with the exception of copper groups concerning glucose) as compared to the control levels. Total leukocytes count (TLC) and differential count was significantly increased in blood of fish (in copper and cold treatments). A non-significant decrease (TLC) was found in fish groups treated with phenol, acidic water, ammonia and starvation. Erythrocytes and platelet counts tended to reduce in all groups except in the hypoxia fish group. Hematocrit values significantly reduced, except in the hypoxia fish group. Concomitant changes in hemoglobin levels were also found. Phenol induced significant decrease in serum lysozyme activity. Hypoxia, cold water, acidic water, and ammonia significantly reduced lysozyme activity. Serum samples collected from fish exposed to copper sulphate showed elevated lysozyme activity compared to the control. Starvation for 14 days significantly reduced lysozyme activity in examined samples. This study have shown that both serum lysozyme activity and serum spontaneous activity (SH) can be determined easily in blood samples drawn from live fish which make them potentially useful markers traits for indirect monitoring of disease resistance in fish (Abdelhamid et al., 2006). Phenolic pollution led to significant decrease in serum concentrations of tri-iodothyronine (T3, 76.3 vs. 31.4 ng/dl) and thyroxin (T4, 8.33 vs. 1.61 ng/dl) hormones but to significant increase in serum levels of total cholesterol (166 vs. 310 mg/dl) and total lipids (1.82 vs 3.26 g/dl). So, phenol is harmful to Nile tilapia (Gad and Saad, 2008). Adam (2007) registered a negative correlation between hematological indices and water quality parameters with moderate regression factors. Relationship between RBC and water quality characteristics was positive and with very low regression factor.
Gradual (but significant) decrease in Hb and PCV values were reported for Nile tilapia by replacing fish meal by gradual percentages of PPM (PPM: consisted of cottonseed, sunflower, canola, sesame and linseed meals) in their diets; whereas the opposite trend was found for the transaminases activity (Soltan et al., 2008). Metwalli (2013) gave the following values for Nile tilapia blood, 6.3-6.5 g/dl total protein, 3.2-3.3.4 g/dl albumin, 7.1-7.7.6 mg/dl urea, 1.13-1.18 mg/dl creatinine, 115-124 u/l AST, and 44.0-46.0 u/l ALT. Fish in poly-culture system (Nile tilapia, silver carp, common carp, and African catfish) fed dried sewage sludge did not negatively affect the blood picture in general, although the significantly elevated values of transaminases activity and triglycerides concentration (Abdelhamid et al., 2014). Abdelhamid et al. (2019) investigated the effect of aflatoxin B (AFB) in the diets of Nile tilapia (Oreochromis niloticus) and the detoxification activity of a commercial anti-mycotoxin ARCAVIT® Bioacid Forte. The experimental period lasted 70 days. Dietary AFB was added at a concentration of 5 ng / g diet. The anti-mycotoxin ARCAVIT® (pro- and prebiotics) is one of the commercial anti-mycotoxins in the local market, which was added at a concentration of 0.5 g/Kg diet. The obtained results showed that AFB is very toxic, even at a low concentration as 5 ng / g diet, for Nile tilapia. It threatens fish hematological and plasma biochemistry parameters. Moreover, the anti-toxic agent (ARCAVIT®) at the tested level did not completely overcome the aflatoxicity symptoms.
- Abdelhamid, A.M., Nemetallah, B.R., Abd Allah, M.A. and Mousa, T.A.E. (2006). Hemolytic activity in blood serum of Oreochromis niloticus under different types of stress. The 3rd Int. Conf. for Develop. and the Env. In the Arab World, March 21 – 23, Assiut Univ., pp: 153 – 169.
- Abdelhamid, A.M.; Refaey, M.M.A., M.E.A.Seden and Zenhom, O.A. (2014b). Effect of different sources and levels of some dietary biological additives on: IV- immunity and haematology of Nile tilapia fish. Egypt. J. Aquat. Biol. & Fish., 18 (1): 49-60.
- Abdelhamid, A. M.; Salem, M.F.I., El-Shebly, A.A. and Sultan, A.S.I. (2019). Is It Possible to Detoxify Aflatoxic Aquafeed? Egyptian Journal of Aquatic Biology & Fisheries, 23 (1): 47 – 63.
- Adam, H.M. (2007). Effect of water quality deterioration on blood profile on Nile tilapia (Oreochromis niloticus). The 11th Conf. of the Egypt. Soc. for the Develop. of Fish. Resor. & Human Health about sustainable use & management of aquatic resources, 28-30 June, Cairo, Abstract No. 116.
- Bagni, M.; Romano, N., finoia, M.G., Abelli, L., Scapigliati, G., Tiscar, P.G., Sarti, M. and Marino, G. (2005). Short-and long –term effects of a dietary β-glucan (Macrogard) and alginic acid (Ergosan) preparation on immune response in sea bass (Dicentrarchus labrax). Fish & Shellfish Immunology, 18: 311-325.
- Daghash, H.A. and Hussein, S.Y. (1999). The role of gonadotrophin releasing hormone (GNRH) injection on spawning, spermiation, some blood constituents and sex hormones in the African catfish, Clarias gariepinus. Egyptian Soc. Anim. Reprod. Fert., Eleventh Annual Congr. Giza 26-28 January, pp: 185-202.
- Decie, S. I. V. and Lewis, S. M. (2006). Practical Hematology. 10th Ed., Churchill Livingstone, London. ISBN: 13: 978- 443, PP: 736.
- Dominguez, M.; Takemura, A., Tsuchiya, M. and Nakamura, S. (2004). Impact of different environmental factors on the circulating immunoglobulin levels in the Nile tilapia, Oreochromis niloticus. Aquaculture, 241: 491-500.
- Ellis, A. E. (1981). Stress and the modulation of defense mechanisms in fish. In “Stress and Fish” (A. D. Pickering; Ed.); pp. 147–169. Academic Press; London
- Gaber, M.M. (2000). Growth response of Nile tilapia fingerlings (Oreochromis niloticus) fed diets containing different levels of clove oil. Egypt. J. Aquat. Biol. & Fish., 4 (1): 1-18.
- Gad, N.S. and Saad, A.S. (2008). Effect of environmental pollution by phenol on some physiological parameters of Oreochromis niloticus. Global Veterinaria, 2 (6): 312-319.
- Hassanen, G.D.I.; Atef, M.A., Abou-Ashour, Abd El-Rahman, H., Abd El-Rahman, S.H. and El-Hammady, A.K.I. (1997). Effect of dietary composition on the performance and some hematological aspects of sea bass (Dicentrarchus labrax). Egypt. J. Aquat. Biol. & Fish., 1 (2): 109-130.
- Hussein, S.Y. and Kobeisy, M.A. (1999). Influence of heat stress on growth performance and some blood constituents of Oreochromis niloticus fed ascorbic acid. Assiut Vet. Med. J., 41 (81): 17-33.
- McGowan, M.W.; Artiss, J.D., Standbergh, D.R. and Zak, B.A. (1983). Peroxidase-coupled method for colorimetric determination of serum triglycerides. Clin. Chem., 29: 538.
- Metwalli, A.A.A. (2013). Effect of partial and total substitution of fish meal with corn gluten meal on growth performance, nutrients utilization and some blood constituents of the Nile tilapia Oreochromis niloticus. Egypt. J. Quat. Biol. & Fish., 17 (1): 91-100.
- Natt, M.P. and Herrick, C.A. (1952). A new blood diluent for counting erythrocytes and leucocytes of the chicken. Poultry Science, 31: 735–738.
- Simide, R.; Richard, S., Pre´vot-D’Alvise, N. Miard, T. and Gaillard, S. (2016). Assessment of the accuracy of physiological blood indicators for the evaluation of stress, health status and welfare in Siberian sturgeon (Acipenser baerii) subject to chronic heat stress and dietary supplementation. Int. Aquat. Res., 8: 121–135.
- Small, B.C. (2004). Effect of isoeugenol sedation on plasma cortisol, glucose, and lactate dynamics in channel catfish Ictalurus punctatus exposed to three stressors. Aquaculture, 238: 469-481.
- Soltan, M.A.; Hanafy, M.A. and Wafa, M.I.A. (2008). Effect of replacing fish meal by a mixture of different plant protein sources in Nile tilapia (Oreochromis niloticus L.) diets. Global Veternaria, 2 (4): 157-164.
- Tietz, N.W. (1986). Textbook of Clinical Chemistry. W.B. Saunders, Philadelphia, 1271.
- Tietz, N.W. (1990). Clinical Guide to Laboratory Tests. 2nd ed. Philadelphia.
- Wotton, I.D. and Freeman, H. (1982). Microanalysis in Medical Biochemistry. Churchill, New York, USA.
Related topics:
Authors:

Recommend
Comment
Share

Would you like to discuss another topic? Create a new post to engage with experts in the community.
Featured users in Aquaculture

Chris Beattie
MSD - Merck Animal Health
Global Head of Aquaculture at Merck Animal Health
United States
United States