Feed additives to improve tibia quality of broilers
Effect of strontium ranelate and cerium oxide addition in the diet on bone quality and expression level of osteocalcin and alkaline phosphatase genes in broiler chicken
Background: In the modern broiler industry, leg and gait disorders are considerable problems. Fast-growing broilers are especially susceptible to bone abnormalities, causing major problems for broiler producers. Strontium ranelate (SrR) has been used successfully for the treatment of osteoporosis in humans. In addition, cerium oxide (CeO) is an anti-stress agent applied in the biological system.
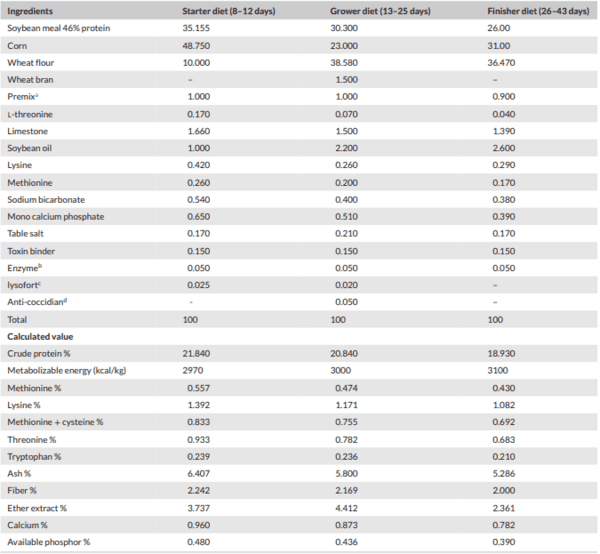
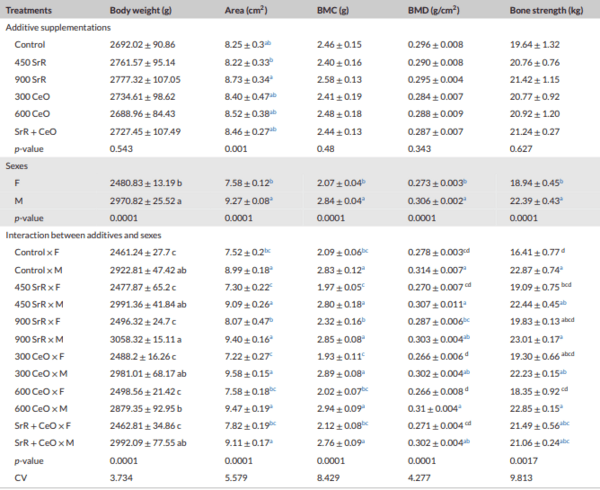
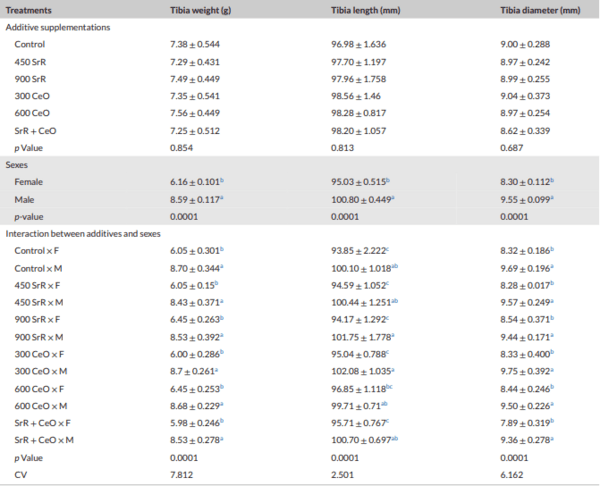
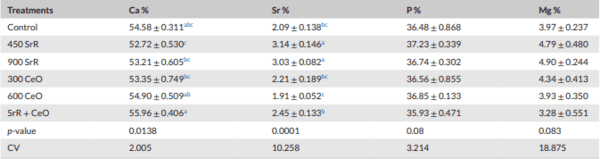
Methods: This study was conducted to investigate the effect of SrR, CeO, and their combinations on tibia quality in broilers. A total of 384 one-day-old Ross chicks were divided into six treatments, with four replicates per treatment (16 birds per replicate). The control group was fed a standard diet, and other groups were fed SrR at levels 450, 900 mg/kg feed, CeO at levels 300 and 600 mg/kg feed and a combination of 450 SrR + 300 CeO mg/kg feed. Bone mineral density (BMD), bone mineral content (BMC), bone strength (BS), tibia area, tibia weight, bone Length, bone diameter, minerals in tibia bone of male broilers, alkaline phosphatase gene (ALP) and osteocalcin gene (OC) in male broilers were analysed.
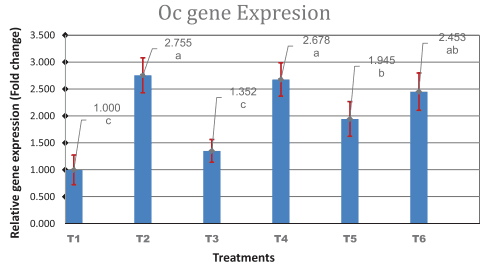
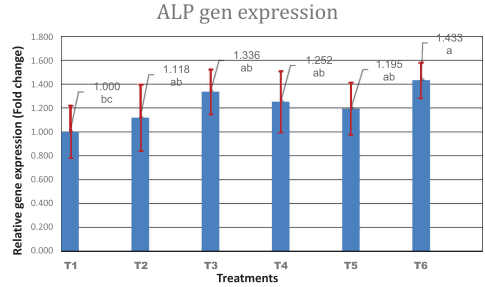
Results: The results showed that the addition of SrR and CeO had no significant influence (p > 0.01) on BMD, BMC, BS, bone weight, bone length and bone diameter. While there was a significant interaction between sex and treatments, especially in the combination group, BS in females significantly (p < 0.01) increased compared to the control group. Generally, females were found to be more responsive to treatments than males. Significant increases in gene expression were noticed in OC with the addition of low levels of SrR and CeO and mixed group compared to the control. The gene expression of ALP was increased significantly only in a combination group compared to the control group.
Conclusion: It is concluded that SrR and CeO can be used as beneficial additives in the feed to improve the tibia quality of broilers.
Keywords: bone; broiler; cerium oxide; gene expression; strontium ranelate.

Adu, O. A., & Olarotimi, O. J. (2020). Quality characteristics of eggs from chickens fed diets containing cerium chloride as rare earth element. Live stock Research for Rural Development, 32, 62. http://www.lrrd.org/lrrd32/ 4/olaro32062.html
Ahmadi, Z., & Ashrafizadeh, M. (2018). Downregulation of osteocalcin gene in chickens treated with lead acetate(II). International Biological and Biomedical Journal, 4(4), 177–182. http://ibbj.org/article-1-189-en.html
Ammann, P. (2006). Strontium ranelate: A physiological approach for an improved bone quality. Bone, 38, 15–18. https://doi.org/10.1016/j.bone. 2005.09.023
Ammann, P., Shen, V., Robin, B., Mauras, Y., Bonjour, J. P., & Rizzoli, R. (2004). Strontium ranelate improves bone resistance by increasing bone mass and improving architecture in intact female rats. Journal of Bone and Mineral Research, 12, 2012–2020. https://doi.org/10.1359/JBMR. 040906
Aveline, P., Cesaro, A., Mazor, M., Best, T., Lespessailles, E., & Toumi, H. (2021). Cumulative effects of strontium ranelate and impact exercise on bone mass in ovariectomized rats. International Journal of Molecular Sciences, 22(6), 3040. https://doi.org/10.3390/ijms22063040
Bain, S. D., Jerome, C., Shen, V., Dupin-Roger, I., & Ammann, P. (2009). Strontium ranelate improves bone strength in ovariectomized rat by positively influencing bone resistance determinants. Osteoporosis International, 20, 1417–1428. https://doi.org/10.1007/s00198-008-0815-8
Bakhit, A., Nobuyuki, K., Kentaro, H., Sonoko, N., Keisuke, N., Masashi, K., Kento, & Takashi, O. (2018). Strontium ranelate promotes odonto- /osteogenic differentiation/mineralization of dental papillae cells in vitro and mineralized tissue formation of the dental pulp in vivo. Scientific Reports, 8(1), 9224. https://doi.org/10.1038/s41598-018-27461- 7
Baracho, M., Nääs, I., Betin, P. S., & Moura, D. (2018). Factors that influence the production, environment, and welfare of broiler chicken: A systematic review. Brazilian Journal of Poultry Science, 20, 617–624. https://doi. org/10.1590/1806-9061-2018-0688
Baracho, M., Nääs, I., Lima, N., Cordeiro, A. F., & Moura, D. (2019). Factors affecting broiler production: A meta-analysis. Brazilian Journal of Poultry Science, 21(3). https://doi.org/10.1590/1806-9061-2019-1052
Bell, D., & Weaver, W. D. (Eds.). (2002). Commercial chicken, meat and egg production Section I General (pp. 135–136). Springer Science+Business Media New York US. https://doi.org/10.1007/978-1-4615-0811- 3
Bishop, S., Fleming, R., Mccormack, H., Flock, D. K., & Whitehead, C. C. (2000). Inheritance of bone characteristics affecting osteoporosis in laying hens. British Poultry Science, 41(1), 33–40. https://doi.org/10.1080/ 00071660086376
Bölükba¸sı, S. C., Al-sagan, A. A., Ürü¸san, H., Erhan, M. K., Durmu¸s, O., & Kurt, N. (2016). Effects of cerium oxide supplementation to laying hen diets on performance, egg quality, some antioxidant enzymes in serum and lipid oxidation in egg yolk. Journal of Animal Physiology and Animal Nutrition, 100(4), 686–693. https://doi.org/10.1111/jpn.12429
Browning, L. C., & Cowieson, A. J. (2013). Effect of vitamin D3 and strontium on performance, nutrient retention, and bone mineral composition in broiler chickens. Animal Production Science, 54(7), 942–949. https:// doi.org/10.1071/AN13091
Browning, L. C., & Cowieson, A. J. (2015). Evaluating vitamin D with graded levels of strontium supplementation on broiler chicken performance and mineral composition. Animal Production Science, 56(1), 70. http://doi.org/ 10.1071/AN14622
CórdobaJover, B., ArceCerezo, A., Ribera, J., Pauta, M., Oró, D., Casals, G., FernándezVaro, G., Casals, E., Puntes, V., Jiménez, W., & MoralesRuiz, M. (2019). Cerium oxide nanoparticles improve liver regeneration after acetaminopheninduced liver injury and partial hepatectomy in rats. Journal of Nanobiotechnology, 17(112). https://doi.org/10.1186/s12951- 019-0544-5
Council, N. R. (1994). Nutrient requirements of poultry. The National Academies Press.
Dahl, S. G., Allain, P., Marie, P. J., Mauras, Y., Boivin, G., Ammann, P., Tsouderos, Y., Delmas, P. D., & Christiansen, C. (2001). Incorporation and distribution of strontium in bone. Bone, 28(4), 446–453. https://doi.org/ 10.1016/S8756-3282(01)00419-7
Doberenz, A. R., Weber, C. W., & Reid, B. L. (1969). Effect of high dietary strontium levels on bone and eggshell calcium and strontium. Calcified Tissue Research, 4, 180–184. https://doi.org/10.1007/BF02279119
Ensrud, K. E., & Crandall, C. J. (2017). Osteoporosis. Annals of Internal Medicine, 167(528), 17–32. https://doi.org/10.7326/AITC201708010
Ferraro, E. F., Carr, R., & Zimmerman, K. (1983). A comparison of the effects of strontium chloride and calcium chloride on alveolar bone. Calcified Tissue International, 35, 258–260. https://doi.org/10.1007/BF02405040
Fleming, R., Mccormack, H., & Whitehead, C. (1998). Bone structure and strength at different ages in laying hens and effects of dietary particulate limestone, vitamin K and ascorbic acid. British Poultry Science, 39, 434–40. https://doi.org/10.1080/00071669889024
Gad, S. C. (2014). Strontium. Encyclopedia of Toxicology, 4, 405. http://doi.org/ 10.1016/B978-0-12-386454-3.00931-3
Golub, E. E., Boesze-Battaglia, K., & Kathleen (2007). The role of alkaline phosphatase in mineralization. Current Opinion in Orthopaedics, 18, 444–448. https://doi.org/10.1097/BCO.0b013e3282630851
Grynpas, M. D., & Marie, P. J. (1990). Effects of low doses of strontium on bone quality and quantity in rats. Bone, 11(5), 313–319. https://doi.org/ 10.1016/8756-3282(90)90086-E
Gulson, B., & Wong, H. (2006). Stable isotopic tracing—a way forward for nanotechnology. Environmental Health Perspectives, 114(10), 1486–1488. https://doi.org/10.1289/ehp.9277
Heckert, E. G., Karakoti, A. S., Seal, S., & Self, W. T. (2008). The role of cerium redox state in the SOD mimetic activity of nanoceria.Biomaterials, 29(270), 5–9. https://doi.org/10.1016/j.biomaterials.2008.03.014
Khan, K., Sevil Kilimci, F., & Kara, M. (2021). Biomechanical tests: Applications and their reliability for the prediction of bone strength in broiler chicken. Veterinary Journal of Mehmet Akif Ersoy University, 6(2), 85–92. https://doi.org/10.24880/maeuvfd.936262
Kierończyk, B., Rawski, M., & Józefiak, D., Świątkiewicz, S. (2017). Infectious and non-infectious factors associated with leg disorders in poultry— A review. Annals of Animal Science, 17(3), 645˗669. https://doi.org/10. 1515/aoas-2016-0098
Komori, T. (2020). Functions of osteocalcin in bone, pancreas, testis, and muscle. International Journal of Molecular Science, 21, 7513. https://doi. org/10.3390/ijms21207513
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods (San Diego, Calif.), 25, 402–408. https://doi.org/10.1006/meth.2001. 1262
Manohar, G. R., Omprakash, A. V., & Kanagaraju, P. (2015). Leg weakness in commercial broiler chicken an overview. International Journal of Science, Environment and Technology, 4(2), 482˗487
Marie, P. J., & Hott, M. (1986). Short-term effects of fluoride and strontium on bone formation and resorption in the mouse. Metabolism, 35(6), 547– 551. https://doi.org/10.1016/0026-0495(86)90013-2
Marie, P. J., Hott, M., Modrowski, D., Pollak, C. D., Guillemain, J., Deloffre, P., & Tsouderos, Y. (1993). An uncoupling agent containing strontium prevents bone loss by depressing bone resorption and maintaining bone formation in estrogen-deficient rats. Journal of Bone and Mineral Research, 8(5), 607–615. https://doi.org/10.1002/jbmr.5650080512
Marie, P. J., Ammann, P., Boivin, G., & Rey, C. (2001). Mechanisms of action and therapeutic potential of strontium in bone. Calcified Tissue International, 69(3), 121–129. https://doi.org/10.1007/s002230010055
Meseret, S. (2016). A review of poultry welfare in conventional production system. Livestock Research for Rural Development, 28, 12.
Meunier, P. J., Roux, C., Ortolani, S., Diaz-Curiel, M., Compston, J., Marquis, P., & Reginster, J. Y. (2009). Effects of long-term strontium ranelate treatment on vertebral fracture risk in postmenopausal women with osteoporosis. Osteoporosis International, 20(10), 1663–1673. https://doi. org/10.1007/s00198-008-0825-6
Meunier, P. J., Roux, C., Seeman, E., Ortolani, S., Badurski, J. E., Spector, T. D., Cannata, J., Balogh, A., Lemmel, E.-M., Pors-Nielsen, S., Rizzoli, R., Genant, H. K., & Reginster, J.-Y. (2004). The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. New England Journal of Medicine, 350, 459–468. https://doi.org/10.1056/ NEJMoa022436
Nakhon, S., Numthuam, S., Charoensook, R., Tartrakoon, W., Incharoen, P., & Incharoen, T. (2019). Growth performance, meat quality, and bonebreaking strength in broilers fed dietary rice hull silicon. Animal Nutrition, 5(2), 152–155. https://doi.org/10.1016/j.aninu.2018.11.003
Nelson, J. R., Eric, B. S., Athrey, G., & Gregory, S. A. (2020). Effects of supplementing yeast fermentate in the feed or drinking water on stress susceptibility, plasma chemistry, cytokine levels, antioxidant status, and stress- and immune-related gene expression of broiler chickens. Poultry Science, 99, 3312–3318. https://doi.org/10.1016/j.psj.2020.03.037
Pilmane, M., Salma-Ancane, K., Loca, D., Locs, J., & Berzina-Cimdina, L. (2017). Strontium and strontium ranelate: Historical review of some of their functions. Materials Science and Engineering: C, 78, 1222–1230. https://doi.org/10.1016/j.msec.2017.05.042
Pounds, J. G., Long, G. J., & Rosen, J. F. (1991). Cellular and molecular toxicity of lead in bone. Environmental Health Perspectives, 91, 17–32. https://doi. org/10.1289/ehp.919117
Rajeshkumar, S., & Naik, P. (2017). Synthesis Synthesis and biomedical applications of cerium oxide nanoparticles—A review. Biotechnology Reports, 17(2018), 1–5. https://doi.org/10.1016/j.btre.2017.11.008
Reka, D., Thavasiappan, V., Selvaraj, P., Arivuchelvan, A., & Visha, P. (2019). Influence of rare earth elements on production performance in post peak layer chickens. Journal of Entomology and Zoology Studies, 7(2), 292–295
Rath, N. C., Balog, J. M., Huff, W. E., Huff, G. R., Kulkarni, G. B., & Tierce, J. F. (1999). Comparative differences in the composition and biomechanical properties of tibiae of seven- and seventy-two-week old male and female broiler breeder chickens. Poultry Science, 78, 1232–1239.
Ross 308 (2012). Ross308 Broiler Performance Manual. Performance Objectives. http://www.en.aviagen.com/assets/Tech_Center/ Ross_Broiler/Ross308BroilerPerfObj2012R1.pdf
Shahnazari, M., Lang, D. H., Fosmire, G. J., Sharkey, N. A., Mitchell, A. D., & Leach, R. M. (2007). Strontium administration in young chickens improves bone volume and architecture but does not enhance bone structural and material strength. Calcified Tissue International, 80, 160–166. https://doi.org/10.1007/s00223-006-0176-2
Shahnazari, M., Sharkey, N. A., Fosmire, G. J., & Leach, R. M. (2006). Effects of strontium on bone strength, density, volume, and microarchitecture in laying hens. Journal of Bone and Mineral Research, 21(11), 1696–703. https://doi.org/10.1359/jbmr.060724
Skoryna, S. C. (Ed.) (1981). Handbook of stable strontium. Skoryna, S. C. and Fuskova, M. Effect of stable strontium supplementation, chapter 35 Plenum Press. 11. pp 593. https://doi.org/10.1007/978-1-4684-3698-3
Suzuki, A., Sekiguchi, S., Asano, S., & Itoh, M. (2008). Pharmacological topics of bone metabolism: Recent advances in the pharmacological management of osteoporosis. Journal of Pharmacological Sciences, 106(4), 530–535. https://doi.org/10.1254/jphs.FM0070218
Tamba, B. I., & Alexa-Stratulat, T. (2017). Trace elements alleviate pain in mice and humans. In R. R. Watson & S. Zibadi (Eds.), Nutritional Modulators of Pain in the Aging Population (199–216). Academic Press. https:// doi.org/10.1016/B978-0-12-805186-3.00017-5
Thorp, B., Whitehead, C., & Rennie, J. (1991). Avian tibial dyschondroplasia: A comparison of the incidence and severity as assessed by gross examination and histopathology. Research in Veterinary Science, 51, 48–54. https://doi.org/10.1016/0034-5288(91)90030-R
Tsai, S. W., Hsu, Y. W., Pan, W. L., & Hsu, F. Y. (2021). The effect of strontium-substituted hydroxyapatite nanofibrous matrix on osteoblast proliferation and differentiation. Membranes, 11(8), 624. https://doi.org/ 10.3390/membranes11080624
Urist, M., & Deutsch, N. (1960). Osteoporosis in the laying hen. Endocrinology, 66, 377–91. https://doi.org/10.1210/endo-66-3-377
Vimalraj, S. (2020). Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene, 754(5), 144855. https://doi.org/ 10.1016/j.gene.2020.144855
Wadkins, C. L., & Peng, C. F. (1981). Strontium metabolism and mechanism of interaction with mineralized tissues. In Skoryna S.C. (Eds.), Handbook of stable strontium. Springer. pp 545–561. https://doi.org/10.1007/978- 1-4684-3698-3_32
Yair, R., Uni, Z., & Shahar, R. (2012). Bone characteristics of late-term embryonic and hatchling broilers: Bone development under extreme growth rate. Poultry Science, 91(10), 2614˗2620. https://doi.org/10.3382/ps. 2012-02244
Zhu, L-L., Zaidi, S., Peng, Y., Peng, Y., Zhou, H., Moonga, BS., Blesius, A., Dupin-Roger, I., Zaidi, M., & Sun, L. (2007). Induction of a program gene expression during osteoblast differentiation with strontium ranelate. Biochemical and Biophysical Research Communications, 355(2), 307–311. https://doi.org/10.1016/j.bbrc.2007.01.120









.jpg&w=3840&q=75)