Introduction
Growth promotional feed additives continue to be an area of emphasis for evaluation, especially in nursery pig diets. One of the classes of feed additives that has gained significant interest is probiotics. Probiotics can be defined as live microorganisms which, when administered in adequate amounts, confer a health benefit on the host (FAO/ WHO, 2001).6 Mechanistically, the modes of action of probiotics are likely to include competitive exclusion of pathogenic bacteria, regulation of local cell-mediated immune responses, increasing antibody production, promotion of epithelial barrier integrity, and the reduction of epithelial cell apoptosis (Ng et al., 2009).7 However, debate still remains within the scientific community on these modes of action.
Among the diverse bacterial species used for probiotics, the nonpathogenic class of Bacillus species are some of the most extensively studied. Of the species, L. plantarum has shown some of the more promising beneficial results on the overall gastrointestinal microbiota of nursery pigs (Pieper et al., 2009; Guerra-Ordaz et al., 2013).8,9 Lactobacillus plantarum is a facultative heterofermentative plant-associated lactic acid bacterium that is tolerant against bile salts and low pH, improving survivability in the GIT (de Vries et al., 2006; da Silva Sabo et al., 2014).10,11 These findings certainly make it a promising bacterial source that could potentially be used in swine diets. However, in the context of these studies, all research has been carried out under highly controlled environments. Research examining its impact in commercial settings is scarce. Thus, the objective of this study was to evaluate the efficacy of Lactobacillus plantarum in nursery pigs in a commercial research facility.
Procedures
The Kansas State University Institutional Animal Care and Use Committee approved the protocol for this experiment. The study was conducted at the Cooperative Research Farm’s Swine Research Nursery (Sycamore, OH), which is owned and managed by Kalmbach Feeds, Inc. Each pen had slatted metal floors and was equipped with a 4-hole stainless steel feeder and one nipple-cup waterer for ad libitum access to feed and water. Pens were 5 × 6 ft to allow 3 ft2 per pig. Nursery rooms were not power washed or disinfected after the previous 6 groups of pigs. A total of 360 pigs (PIC C-29 × 359, initially 13.1 lb BW) with 10 pigs per pen and 9 replications per treatment were used in a 42-d trial.
Pigs were weaned at approximately 16 to 20 d of age and allotted to pens based on initial BW and gender to 1 of 4 dietary treatments in a completely randomized design. Pigs and feeders were weighed every 7 d of the trial to determine ADG, ADFI, and F/G.
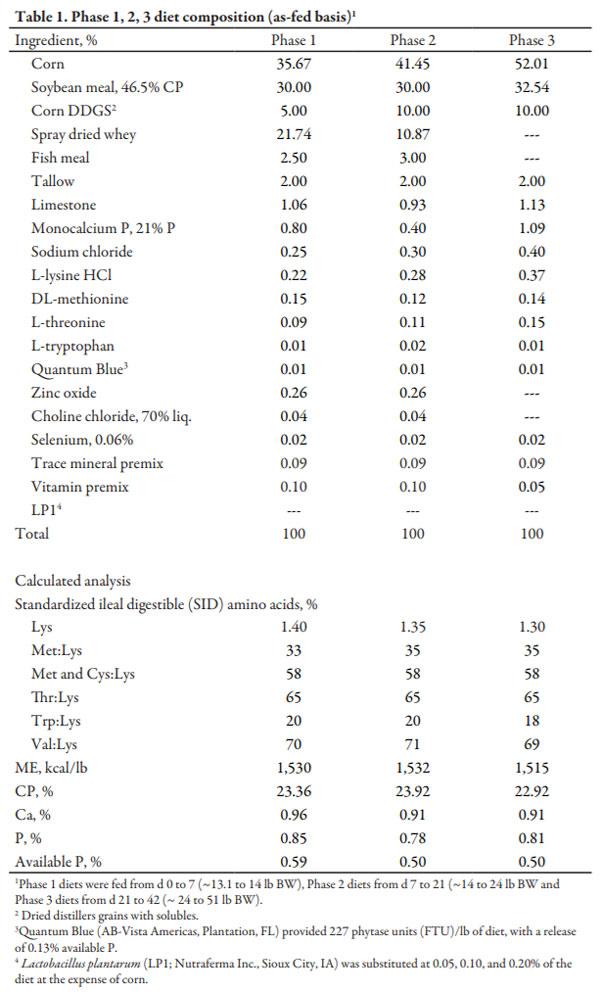
Experimental diets were fed in three phases (Table 1). Phase 1 was fed from d 0 to 7 d. The second phase was fed from d 7 to 21 (~ 25 lb BW), while phase 3 was fed from d 21 to 42 post-weaning. Treatment diets were formulated to include 0, 0.05, 0.10, or 0.20% of Lactobacillus plantarum product (LP1; Nutraferma Inc., Sioux City, IA). All treatment diets within phase were formulated to similar nutrient levels. All experimental diets were fed in pellet form.
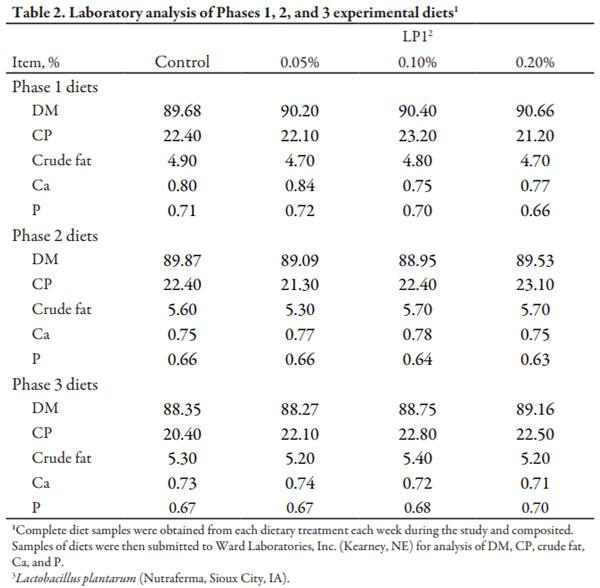
Complete diet samples were collected and analyzed for DM, CP, crude fat, Ca, and P (Table 2).
Data were analyzed using the PROC GLIMIX procedure in SAS (SAS Institute, Inc., Cary, NC) with pen as the experimental unit. Dietary treatment served as the fixed effect in the model. Linear and quadratic responses were determined for increasing LP1. A P value ≤ 0.05 was considered significant and 0.05 < P ≤ 0.10 was considered a tendency.
Results and Discussion
Chemical analysis of complete diets revealed that analyzed values were similar to calculated values (Table 2).
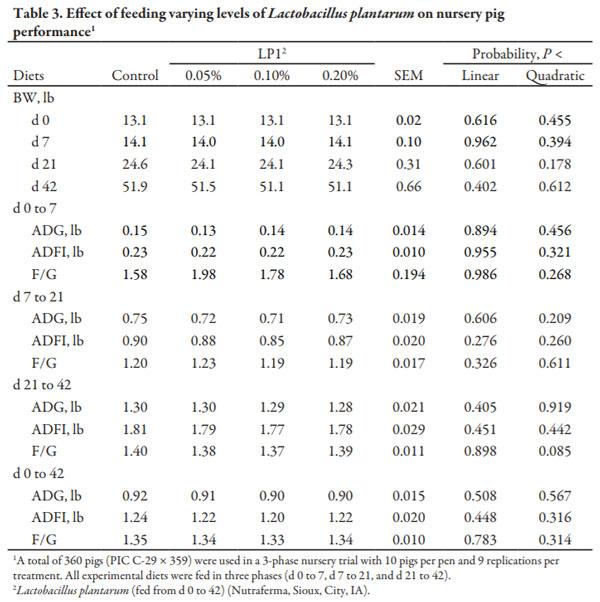
From d 0 to 7 (Phase 1) and d 7 to 21 (Phase 2), there were no differences in growth performance observed among dietary treatments (Table 3). From d 21 to 42 (Phase 3), ADG and ADFI were not influenced by treatment; however, F/G tended to improve (quadratic, P = 0.085) with increasing LP1 up to 0.10%. Overall (d 0 to 42), there were no differences in growth performance detected between dietary treatments.
The addition of up to 0.20% LP1 used in this study, resulted in no improvements in overall ADG, ADFI, or F/G. Additional research should be conducted to determine if LP1 elicits a response in other diet formulation or health statuses. One possible explanation could be attributed to the fact that the overall health status of the pigs throughout this experiment was very good. This occurred although the room that they were placed into had not been power washed or disinfected after the previous 6 groups of pigs.
This article was originally published in Kansas Agricultural Experiment Station Research Reports: Vol. 2: Iss. 8. https://doi.org/10.4148/2378-5977.1292. This is an Open Access article is licensed under a Creative Commons Attribution 4.0 License.