Effects of mineral methionine hydroxy analog chelate in sow diets on epigenetic modification and growth of progeny
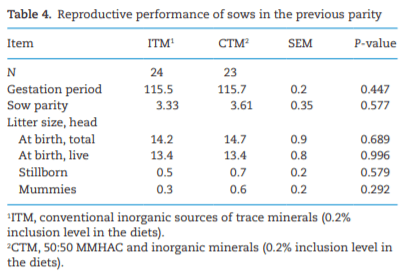
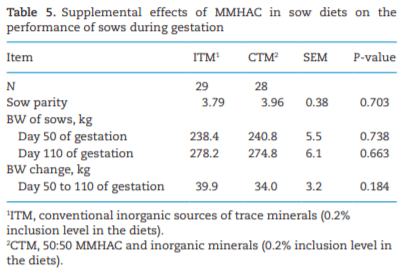
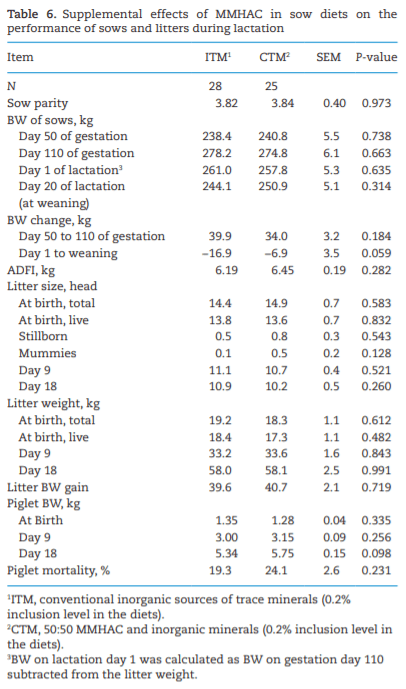
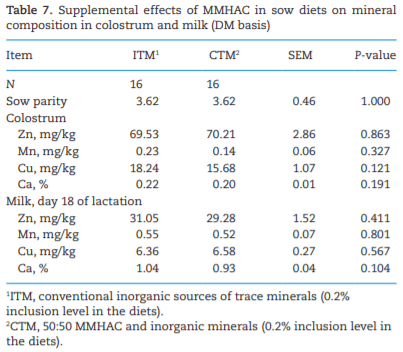
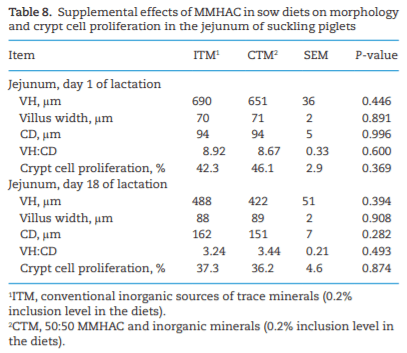
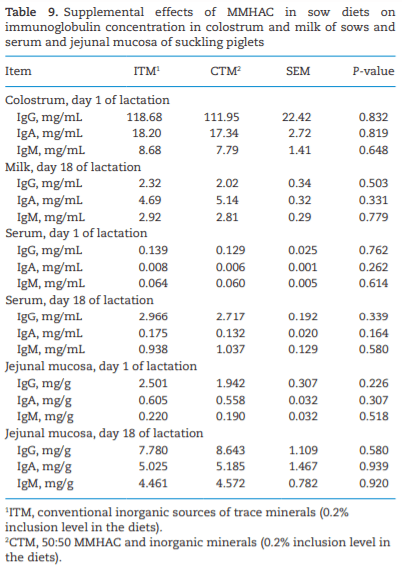
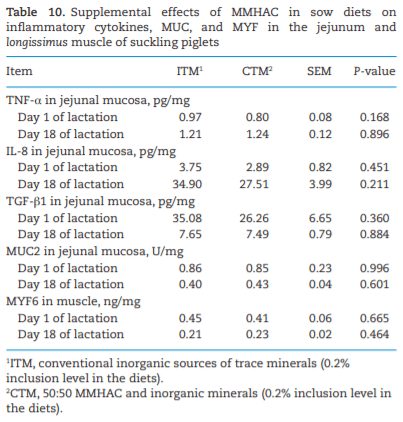
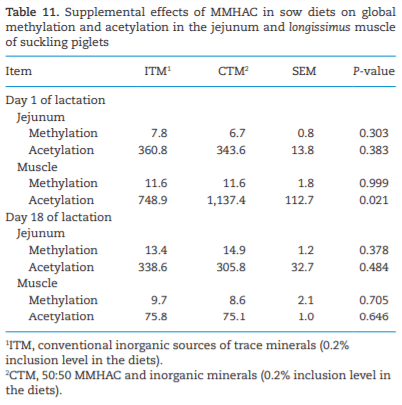
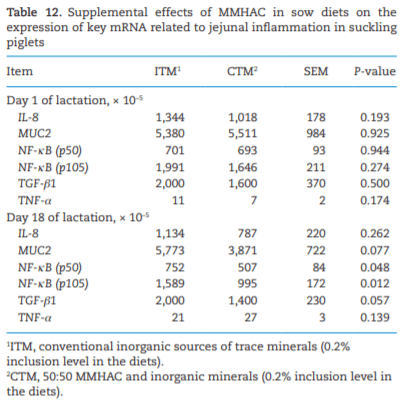
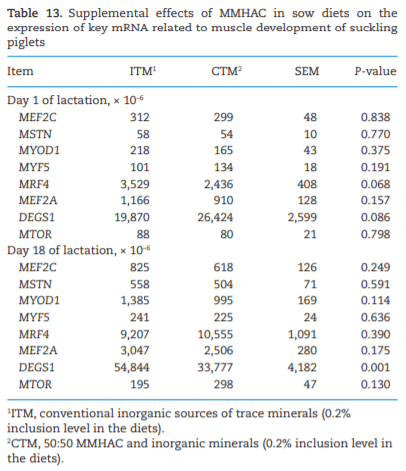
The study was conducted to determine the effects of mineral methionine hydroxy analog chelate (MMHAC) partially replacing inorganic trace minerals in sow diets on epigenetic and transcriptional changes in the muscle and jejunum of progeny. The MMHAC is zinc (Zn), manganese (Mn), and copper (Cu) chelated with methionine hydroxy analog (Zn-, Mn-, and Cu-methionine hydroxy analog chelate [MHAC]). On day 35 of gestation, 60 pregnant sows were allotted to two dietary treatments in a randomized completed block design using parity as a block: 1) ITM: inorganic trace minerals with zinc sulfate (ZnSO4), manganese oxide (MnO), and copper sulfate (CuSO4) and 2) CTM: 50% of ITM was replaced with MMHAC (MINTREX trace minerals, Novus International Inc., St Charles, MO). Gestation and lactation diets were formulated to meet or exceed NRC requirements. On days 1 and 18 of lactation, milk samples from 16 sows per treatment were collected to measure immunoglobulins (immunoglobulin G, immunoglobulin A, and immunoglobulin M) and micromineral concentrations. Two pigs per litter were selected to collect blood to measure the concentration of immunoglobulins in the serum, and then euthanized to collect jejunal mucosa, jejunum tissues, and longissimus muscle to measure global deoxyribonucleic acid methylation, histone acetylation, cytokines, and jejunal histomorphology at birth and day 18 of lactation. Data were analyzed using Proc MIXED of SAS. Supplementation of MMHAC tended to decrease (P = 0.059) body weight (BW) loss of sows during lactation and tended to increase (P = 0.098) piglet BW on day 18 of lactation. Supplementation of MMHAC increased (P < 0.05) global histone acetylation and tended to decrease myogenic regulatory factor 4 messenger ribonucleic acid (mRNA; P = 0.068) and delta 4-desaturase sphingolipid1 (DEGS1) mRNA (P = 0.086) in longissimus muscle of piglets at birth. Supplementation of MMHAC decreased (P < 0.05) nuclear factor kappa B mRNA in the jejunum and DEGS1 mRNA in longissimus muscle and tended to decrease mucin-2 (MUC2) mRNA (P = 0.057) and transforming growth factor-beta 1 (TGF-β1) mRNA (P = 0.057) in the jejunum of piglets on day 18 of lactation. There were, however, no changes in the amounts of tumor necrosis factor-alpha, interleukin-8, TGF-β, MUC2, and myogenic factor 6 in the tissues by MMHAC. In conclusion, maternal supplementation of MMHAC could contribute to histone acetylation and programming in the fetus, which potentially regulates intestinal health and skeletal muscle development of piglets at birth and weaning, possibly leading to enhanced growth of their piglets.
Key words: chelated minerals, growth, histone acetylation, intestinal health, piglets, sows
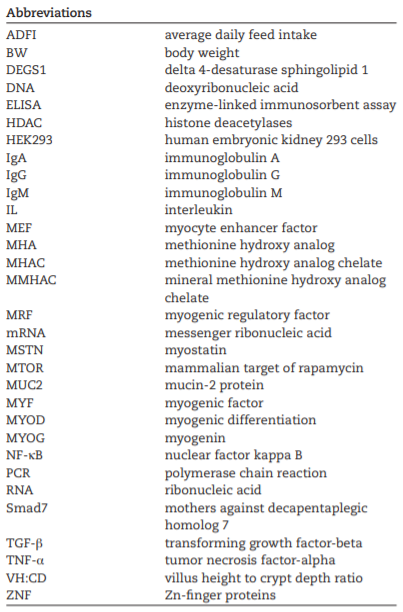
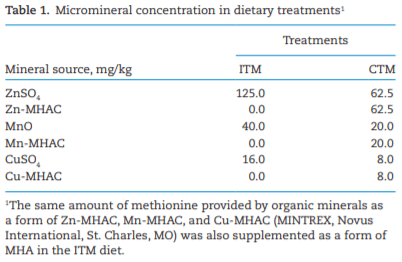
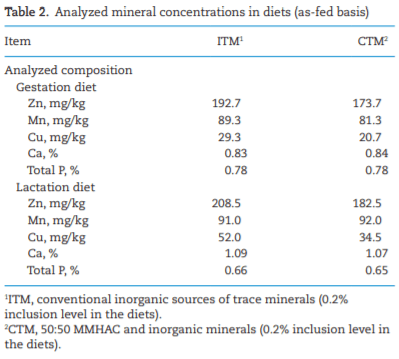
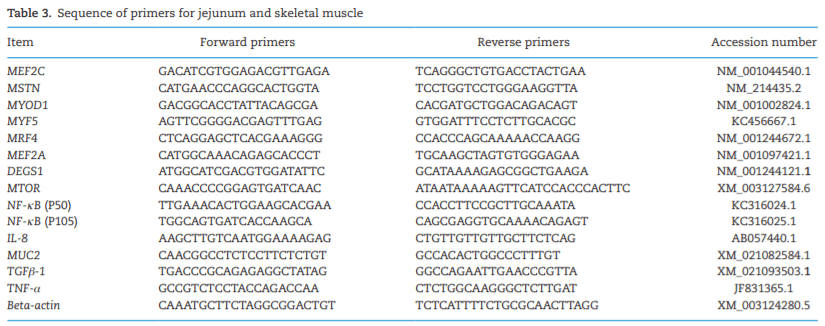
AAFCO. 2018. Official Publication of Association of American Feed Control Officials. Champaign (IL): AAFCO.
Anderson, O. S., K. E. Sant, and D. C. Dolinoy. 2012. Nutrition and epigenetics: an interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J. Nutr. Biochem. 23:853–859. doi:10.1016/j.jnutbio.2012.03.003
Barbarroja, N., S. Rodriguez-Cuenca, H. Nygren, A. Camargo, A. Pirraco, J. Relat, I. Cuadrado, V. Pellegrinelli, G. Medina-Gomez, C. Lopez-Pedrera, et al. 2015. Increased dihydroceramide/ceramide ratio mediated by defective expression of degs1 impairs adipocyte differentiation and function. Diabetes 64:1180–1192. doi:10.2337/db14-0359
Burdge, G. C., and K. A. Lillycrop. 2010. Nutrition, epigenetics, and developmental plasticity: implications for understanding human disease. Annu. Rev. Nutr. 30:315–339. doi:10.1146/ annurev.nutr.012809.104751
Cassandri, M., A. Smirnov, F. Novelli, C. Pitolli, M. Agostini, M. Malewicz, G. Melino, and G. Raschellà. 2017. Zinc-finger proteins in health and disease. Cell Death Discov. 3:17071. doi:10.1038/cddiscovery.2017.71
Chen, J., G. Tellez, J. D. Richards, and J. Escobar. 2015. Identification of potential biomarkers for gut barrier failure in broiler chickens. Front. Vet. Sci. 2:14. doi:10.3389/fvets.2015.00014
Chen, H., S. Zhang, I. Park, and S. W. Kim. 2017. Impacts of energy feeds and supplemental protease on growth performance, nutrient digestibility, and gut health of pigs from 18 to 45 kg body weight. Anim. Nutr. 3:359–365. doi:10.1016/j. aninu.2017.09.005
Delage, B., and R. H. Dashwood. 2008. Dietary manipulation of histone structure and function. Annu. Rev. Nutr. 28:347–366. doi:10.1146/annurev.nutr.28.061807.155354
Duarte, M. E., F. X. Zhou, W. M. Dutra Jr, and S. W. Kim. 2019. Dietary supplementation of xylanase and protease on growth performance, digesta viscosity, nutrient digestibility, immune and oxidative stress status, and gut health of newly weaned pigs. Anim. Nutr. 5:351–358. doi:10.1016/j. aninu.2019.04.005
Dwyer, C. M., N. C. Stickland, and J. M. Fletcher. 1994. The influence of maternal nutrition on muscle fiber number development in the porcine fetus and on subsequent postnatal growth. J. Anim. Sci. 72:911–917. doi:10.2527/1994.724911x
Edfors, F., F. Danielsson, B. M. Hallström, L. Käll, E. Lundberg, F. Pontén, B. Forsström, and M. Uhlén. 2016. Gene-specific correlation of RNA and protein levels in human cells and tissues. Mol. Syst. Biol. 12:883. doi:10.15252/msb.20167144
Farmer, C. 2018. Nutritional impact on mammary development in pigs: a review. J. Anim. Sci. 96:3748–3756. doi:10.1093/jas/ sky243
Fell, B. F., C. F. Mills, and R. Boyne. 1965. Cytochrome oxidase deficiency in the motor neurons of copper-deficient lambs: a histochemical study. Res. Vet. Sci. 6:170–180. doi:10.1016/ S0034-5288(18)34752-0
Gartstein, M. A., and M. K. Skinner. 2018. Prenatal influences on temperament development: the role of environmental epigenetics. Dev. Psychopathol. 30:1269–1303. doi:10.1017/ S0954579417001730
Gooneratne, S. R., and D. A. Christensen. 1989. A survey of maternal copper status and fetal tissue copper concentrations in Saskatchewan bovine. Can. J. Anim. Sci. 69:141–150. doi:10.4141/cjas89-017
Gorelik, L., and R. A. Flavell. 2000. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity 12:171–181. doi:10.1016/ s1074-7613(00)80170-3
Gorelik, L., and R. A. Flavell. 2002. Transforming growth factor-beta in T-cell biology. Nat. Rev. Immunol. 2:46–53. doi:10.1038/ nri704
Grace, N. D., J. H. Watkinson, and P. L. Martinson. 1986. Accumulation of minerals by the foetus(es) and conceptus of single- and twin-bearing ewes. New Zeal. J. Agric. Res. 29:207– 222. doi:10.1080/00288233.1986.10426974
Gresham, J. D., S. R. McPeake, J. K. Bernard, M. J. Riemann, R. W. Wyatt, and H. H. Henderson. 1994. Prediction of live and carcass characteristics of market hogs by use of a single longitudinal ultrasonic scan. J. Anim. Sci. 72:1409–1416. doi:10.2527/1994.7261409x.
Grégoire, S., L. Xiao, J. Nie, X. Zhang, M. Xu, J. Li, J. Wong, E. Seto, and X. J. Yang. 2007. Histone deacetylase 3 interacts with and deacetylates myocyte enhancer factor 2. Mol. Cell. Biol. 27:1280–1295. doi:10.1128/MCB.00882-06
Haren, M. T., A. M. Siddiqui, H. J. Armbrecht, R. T. Kevorkian, M. J. Kim, M. J. Haas, A. Mazza, V. B. Kumar, M. Green, W. A. Banks, et al. 2011. Testosterone modulates gene expression pathways regulating nutrient accumulation, glucose metabolism and protein turnover in mouse skeletal muscle. Int. J. Androl. 34:55–68. doi:10.1111/j.1365-2605.2010.01061.x
Hinterberger, T. J., D. A. Sassoon, S. J. Rhodes, and S. F. Konieczny. 1991. Expression of the muscle regulatory factor MRF4 during somite and skeletal myofiber development. Dev. Biol. 147:144– 156. doi:10.1016/s0012-1606(05)80014-4
Hollis, G. R., S. D. Carter, T. R. Cline, T. D. Crenshaw, G. L. Cromwell, G. M. Hill, S. W. Kim, A. J. Lewis, D. C. Mahan, P. S. Miller, et al. 2005. Effects of replacing pharmacological levels of dietary zinc oxide with lower dietary levels of various organic zinc sources for weanling pigs. J. Anim. Sci. 83:2123–2129. doi:10.2527/2005.8392123x
Horodyska, J., K. Wimmers, H. Reyer, N. Trakooljul, A. M. Mullen, P. G. Lawlor, and R. M. Hamill. 2018. RNA-seq of muscle from pigs divergent in feed efficiency and product quality identifies differences in immune response, growth, and macronutrient and connective tissue metabolism. BMC Genomics 19:791. doi:10.1186/s12864-018-5175-y
Hostetler, C. E., R. L. Kincaid, and M. A. Mirando. 2003. The role of essential trace elements in embryonic and fetal development in livestock. Vet. J. 166:125–139. doi:10.1016/ s1090-0233(02)00310-6
Ibeagha-Awemu, E. M., and X. Zhao. 2015. Epigenetic marks: regulators of livestock phenotypes and conceivable sources of missing variation in livestock improvement programs. Front. Genet. 6:302. doi:10.3389/fgene.2015.00302
Jiao, Q., A. M. Pruznak, D. Huber, T. C. Vary, and C. H. Lang. 2009. Castration differentially alters basal and leucine-stimulated tissue protein synthesis in skeletal muscle and adipose tissue. Am. J. Physiol. Endocrinol. Metab. 297:E1222-32. doi:10.1152/ajpendo.00473.2009
Johansson, M. E. V., J. M. Holmén Larsson, and G. C. Hansson. 2011. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc. Natl. Acad. Sci. U. S. A. 108:4659– 4665. doi:10.1073/pnas.1006451107
Jorgensen, B. G., and S. Ro. 2019. Role of DNA methylation in the development and differentiation of intestinal epithelial cells and smooth muscle cells. J. Neurogastroenterol. Motil. 25:377– 386. doi:10.5056/jnm19077
Kim, S. W., and J. A. Hansen. 2013. Feed formulation and feeding program. In: Chiba, L., editor, Sustainable swine nutrition. Hoboken, New Jersey: Wiley-Blackwell; p. 217–228.
Kim, S. W., W. L. Hurley, I. K. Han, H. H. Stein, and R. A. Easter. 1999. Effect of nutrient intake on mammary gland growth in lactating sows. J. Anim. Sci. 77:3304–3315. doi:10.2527/1999.77123304x
Kim, S. W., W. L. Hurley, G. Wu, and F. Ji. 2009. Ideal amino acid balance for sows during gestation and lactation. J. Anim. Sci. 87:123–132. doi:10.2527/jas.2008-1452.
Kim, S. W., and G. Wu. 2009. Regulatory role for amino acids in mammary gland growth and milk synthesis. Amino Acids 37:89–95. doi:10.1007/s00726-008-0151-5
Kulkarni, A. B., and S. Karlsson. 1993. Transforming growth factor-beta 1 knockout mice. A mutation in one cytokine gene causes a dramatic inflammatory disease. Am. J. Pathol. 143:3–9.
Lezzi, S., G. Cossu, C. Nervi, V. Sartorelli, and P. L. Puri. 2002. Stage-specific modulation of skeletal myogenesis by inhibitors of nuclear deacetylases. Proc. Natl. Acad. Sci. U. S. A. 99:7757– 7762. doi:10.1073/pnas.112218599
Li, C., S. Guo, J. Gao, Y. Guo, E. Du, Z. Lv, and B. Zhang. 2015. Maternal high-zinc diet attenuates intestinal inflammation by reducing DNA methylation and elevating H3K9 acetylation in the A20 promoter of offspring chicks. J. Nutr. Biochem. 26:173–183. doi:10.1016/j.jnutbio.2014.10.005
Liao, P., X. Shu, M. Tang, B. Tan, and Y. Yin. 2018. Effect of dietary copper source (inorganic vs. chelated) on immune response, mineral status, and fecal mineral excretion in nursery piglets. Food Agric. Immunol. 29:548–563. doi:10.1080/09540105.2017.14 16068
Liu, Y., A. Beyer, and R. Aebersold. 2016. On the dependency of cellular protein levels on mRNA abundance. Cell 165:535–550. doi:10.1016/j.cell.2016.03.014
Liu, Y., Y. L. Ma, J. M. Zhao, M. Vazquez-Añón, and H. H. Stein. 2014. Digestibility and retention of zinc, copper, manganese, iron, calcium, and phosphorus in pigs fed diets containing inorganic or organic minerals. J. Anim. Sci. 92:3407–3415. doi:10.2527/jas.2013-7080.
Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. doi:10.1006/ meth.2001.1262
Mahan, D. C., M. Azain, T. D. Crenshaw, G. L. Cromwell, C. R. Dove, S. W. Kim, M. D. Lindemann, P. S. Miller, J. E. Pettigrew, H. H. Stein, et al. 2014. Supplementation of organic and inorganic selenium to diets using grains grown in various regions of the United States with differing natural Se concentrations and fed to grower-finisher swine. J. Anim. Sci. 92:4991–4997. doi:10.2527/jas.2014-7735
Mateo, R. D., J. E. Spallholz, R. Elder, I. Yoon, and S. W. Kim. 2007. Efficacy of dietary selenium sources on growth and carcass characteristics of growing-finishing pigs fed diets containing high endogenous selenium. J. Anim. Sci. 85:1177–1183. doi:10.2527/jas.2006-067
Mayorga, E. J., S. K. Kvidera, E. A. Horst, M. Al-Qaisi, M. J. Dickson, J. T. Seibert, S. Lei, A. F. Keating, J. W. Ross, R. P. Rhoads, et al. 2018. Effects of zinc amino acid complex on biomarkers of gut integrity and metabolism during and following heat stress or feed restriction in pigs. J. Anim. Sci. 96:4173–4185. doi:10.1093/ jas/sky293
McPherson, R. L., F. Ji, G. Wu, and S. W. Kim. 2004. Fetal growth and compositional changes of fetal tissues in the pigs. J. Anim. Sci. 82:2534–2540. doi:10.2527/2004.8292534x
Minetti, G. C., C. Colussi, R. Adami, C. Serra, C. Mozzetta, V. Parente, S. Fortuni, S. Straino, M. Sampaolesi, M. Di Padova, et al. 2006. Functional and morphological recovery of dystrophic muscles in mice treated with deacetylase inhibitors. Nat. Med. 12:1147–1150. doi:10.1038/ nm1479
Moncaut, N., P. W. Rigby, and J. J. Carvajal. 2013. Dial M(RF) for myogenesis. FEBS J. 280:3980–3990. doi:10.1111/febs.12379
Moore, L. D., T. Le, and G. Fan. 2013. DNA methylation and its basic function. Neuropsychopharmacology 38:23–38. doi:10.1038/ npp.2012.112
Moresi, V., N. Marroncelli, D. Coletti, and S. Adamo. 2015. Regulation of skeletal muscle development and homeostasis by gene imprinting, histone acetylation and microRNA. Biochim. Biophys. Acta 1849:309–316. doi:10.1016/j. bbagrm.2015.01.002
Moretti, I., S. Ciciliot, K. A. Dyar, R. Abraham, M. Murgia, L. Agatea, T. Akimoto, S. Bicciato, M. Forcato, P. Pierre, et al. 2016. MRF4 negatively regulates adult skeletal muscle growth by repressing MEF2 activity. Nat. Commun. 7:12397. doi:10.1038/ ncomms12397
Murray, R. L., W. Zhang, M. Iwaniuk, E. Grilli, and C. H. Stahl. 2018. Dietary tributyrin, an HDAC inhibitor, promotes muscle growth through enhanced terminal differentiation of satellite cells. Physiol. Rep. 6:e13706. doi:10.14814/phy2.13706
Neurath, M. F., C. Becker, and K. Barbulescu. 1998. Role of NF-kappaB in immune and inflammatory responses in the gut. Gut 43:856–860. doi:10.1136/gut.43.6.856
Nie, Y., S. Cai, R. Yuan, S. Ding, X. Zhang, L. Chen, Y. Chen, and D. Mo. 2019. Zfp422 promotes skeletal muscle differentiation by regulating EphA7 to induce appropriate myoblast apoptosis. Cell Death Differ 27:1644–1659. doi:10.1038/ s41418-019-0448-9
NRC. 2012. Nutrient requirements of swine. 11h rev. ed. Washington (DC): The National Academies Press.
Otsuji, T. G., Y. Kurose, H. Suemori, M. Tada, and N. Nakatsuji. 2012. Dynamic link between histone H3 acetylation and an increase in the functional characteristics of human ESC/ iPSC-derived cardiomyocytes. PLoS One. 7:e45010. doi:10.1371/ journal.pone.0045010
Pavlath, G. K., J. A. Dominov, K. M. Kegley, and J. B. Miller. 2003. Regeneration of transgenic skeletal muscles with altered timing of expression of the basic helix-loop-helix muscle regulatory factor MRF4. Am. J. Pathol. 162:1685–1691. doi:10.1016/S0002-9440(10)64303-9
Perera, F., and J. Herbstman. 2011. Prenatal environmental exposures, epigenetics, and disease. Reprod. Toxicol. 31:363– 373. doi:10.1016/j.reprotox.2010.12.055
Prasad, A. S., B. Bao, F. W. Beck, O. Kucuk, and F. H. Sarkar. 2004. Antioxidant effect of zinc in humans. Free Radic. Biol. Med. 37:1182–1190. doi:10.1016/j.freeradbiomed.2004.07.007
Ropka-Molik, K., A. Bereta, K. Zukowski, M. Tyra, K. Piórkowska, G. Zak, and M. Oczkowicz. 2018. Screening for candidate genes related with histological microstructure, meat quality and carcass characteristic in pig based on RNA-seq data. AsianAustralas. J. Anim. Sci. 31:1565–1574. doi:10.5713/ajas.17.0714
Salmon, M., G. K. Owens, and Z. E. Zehner. 2009. Over-expression of the transcription factor, ZBP-89, leads to enhancement of the C2C12 myogenic program. Biochim. Biophys. Acta 1793:1144–1155. doi:10.1016/j.bbamcr.2009.01.019
Schiaffino, S., K. A. Dyar, and E. Calabria. 2018. Skeletal muscle mass is controlled by the MRF4–MEF2 axis. Curr. Opin. Clin. Nutr. Metab. Care 21:164–167. doi:10.1097/MCO.0000000000000456
Shannon, M. C., and G. M. Hill. 2019. Trace mineral supplementation for the intestinal health of young monogastric animals. Front. Vet. Sci. 6:73. doi:10.3389/fvets.2019.00073
Shen, Y. B., J. A. Carroll, I. Yoon, R. D. Mateo, and S. W. Kim. 2011. Effects of supplementing Saccharomyces cerevisiae fermentation product in sow diets on performance of sows and nursing piglets. J. Anim. Sci. 89:2462–2471. doi:10.2527/ jas.2010-3642
Shen, Y. B., X. S. Piao, S. W. Kim, L. Wang, P. Liu, I. Yoon, and Y. G. Zhen. 2009. Effects of yeast culture supplementation on growth performance, intestinal health, and immune response of nursery pigs. J. Anim. Sci. 87:2614–2624. doi:10.2527/ jas.2008-1512
Shifera, A. S. 2010. Proteins that bind to IKKgamma (NEMO) and down-regulate the activation of NF-kappaB. Biochem. Biophys. Res. Commun. 396:585–589. doi:10.1016/j.bbrc.2010.05.012
Shiou, S. R., Y. Yu, Y. Guo, M. Westerhoff, L. Lu, E. O. Petrof, J. Sun, and E. C. Claud. 2013. Oral administration of transforming growth factor-β1 (TGF-β1) protects the immature gut from injury via Smad protein-dependent suppression of epithelial nuclear factor κB (NF-κB) signaling and proinflammatory cytokine production. J. Biol. Chem. 288:34757–34766. doi:10.1074/jbc.M113.503946
Sincennes, M. C., C. E. Brun, and M. A. Rudnicki. 2016. Concise Review: Epigenetic regulation of myogenesis in health and disease. Stem Cells Transl. Med. 5:282–290. doi:10.5966/ sctm.2015-0266
Sterner, D. E., and S. L. Berger. 2000. Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 64:435– 459. doi:10.1128/mmbr.64.2.435-459.2000
Tarleton, B. J., A. A. Wiley, and F. F. Bartol. 2001. Neonatal estradiol exposure alters uterine morphology and endometrial transcriptional activity in prepubertal gilts. Domest. Anim. Endocrinol. 21:111–125. doi:10.1016/s0739-7240(01)00106-0
Troncone, E., I. Marafini, C. Stolfi, and G. Monteleone. 2018. Transforming growth factor-β1/Smad7 in intestinal immunity, inflammation, and cancer. Front. Immunol. 9:1407. doi:10.3389/fimmu.2018.01407
Turner, B. M. 2000. Histone acetylation and an epigenetic code. Bioessays. 22:836–845. doi:10.1002/1521- 1878(200009)22:9<836::AID-BIES9>3.0.CO;2-X
Vogel, C., and E. M. Marcotte. 2012. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 13:227–232. doi:10.1038/nrg3185
Wang, S., P. Xia, Y. Chen, Y. Qu, Z. Xiong, B. Ye, Y. Du, Y. Tian, Z. Yin, Z. Xu, et al. 2017. Regulatory innate lymphoid cells control innate intestinal inflammation. Cell 171:201–216.e18. doi:10.1016/j.cell.2017.07.027
Waterland, R. A. 2006. Assessing the effects of high methionine intake on DNA methylation. J. Nutr. 136(6 Suppl):1706S–1710S. doi:10.1093/jn/136.6.1706S
Wiedeman, A. M., S. I. Barr, T. J. Green, Z. Xu, S. M. Innis, and D. D. Kitts. 2018. Dietary choline intake: current state of knowledge across the life cycle. Nutrients 10:1513. doi:10.3390/nu10101513
Wigmore, P. M., and N. C. Stickland. 1983. Muscle development in large and small pig fetuses. J. Anat. 137 (Pt 2):235–245.
Wu, G., F. W. Bazer, T. A. Cudd, C. J. Meininger, and T. E. Spencer. 2004. Maternal nutrition and fetal development. J. Nutr. 134:2169–2172. doi:10.1093/jn/134.9.2169
Yan, X., M. J. Zhu, M. V. Dodson, and M. Du. 2013. Developmental programming of fetal skeletal muscle and adipose tissue development. J. Genomics 1:29–38. doi:10.7150/jgen.3930
Zhang, L. B., and Y. M. Guo. 2008. Effects of liquid dl-2-hydroxy-4-methylthio butanoic acid on growth performance and immune responses in broiler chickens. Poult. Sci. 87:1370– 1376. doi:10.3382/ps.2007-00366
Zhang, S., J. Heng, H. Song, Y. Zhang, X. Lin, M. Tian, F. Chen, and W. Guan. 2019. Role of maternal dietary protein and amino acids on fetal programming, early neonatal development, and lactation in swine. Animals (Basel). 9:19. doi:10.3390/ani9010019
Zhang, B., Y. Shao, D. Liu, P. Yin, Y. Guo, and J. Yuan. 2012. Zinc prevents Salmonella enterica serovar Typhimurium-induced loss of intestinal mucosal barrier function in broiler chickens. Avian Pathol. 41:361–367. doi:10.1080/03079457.2012.692155
Zhang, F., W. Zheng, R. Guo, and W. Yao. 2017. Effect of dietary copper level on the gut microbiota and its correlation with serum inflammatory cytokines in Sprague-Dawley rats. J. Microbiol. 55:694–702. doi:10.1007/s12275-017-6627-9
Zhao, J., G. Allee, G. Gerlemann, L. Ma, M. I. Gracia, D. Parker, M. Vazquez-Anon, and R. J. Harrell. 2014. Effects of a chelated copper as growth promoter on performance and carcass traits in pigs. Asian-Australas. J. Anim. Sci. 27:965–973. doi:10.5713/ ajas.2013.13416
Zhao, J., A. F. Harper, M. J. Estienne, K. E. Webb Jr, A. P. McElroy, and D. M. Denbow. 2007. Growth performance and intestinal morphology responses in early weaned pigs to supplementation of antibiotic-free diets with an organic copper complex and spray-dried plasma protein in sanitary and nonsanitary environments. J. Anim. Sci. 85:1302–1310. doi:10.2527/jas.2006-434
Zhu, Y., L. Lu, X. Liao, W. Li, L. Zhang, C. Ji, X. Lin, H.-C. Liu, J. Odle, and X. Luo. 2017. Maternal dietary manganese protects chick embryos against maternal heat stress via epigenetic-activated antioxidant and anti-apoptotic abilities. Oncotarget 8:89665–89680. doi:10.18632/oncotarget. 20804











.jpg&w=3840&q=75)