Nutritionally induced cellular signals that affect skeletal integrity in swine
Published: July 29, 2014
By: Thomas Crenshaw, DVM, PhD. Department of Animal Science, University of Wisconsin-Madison
Introduction
Insights into skeletal integrity gained from an accidental omission of vitamin D. A recent escalation in lameness and mortality issues in the U.S. swine industry (Madson et al., 2012) were attributed to hypovitaminosis D. An explanation for the abrupt increase in clinical cases of vitamin D deficiency in commercial swine is not apparent. The swine industry has alleviated some of these issues by modifications in vitamin D supplements, but problems still persist (Arnold et al., 2014). Although the number of cases has subsided, the issues spurred interest and questions about bone composition and methods used to accurately assess skeletal integrity. Necropsy reports that describe fractures, callous ribs, and “rubbery” bones may reflect extreme conditions of a nutrient deficiency. More definitive descriptors are needed as guidelines to establish a balance between nutrient inputs required for animal well-being and the environmental issues that often pressure nutrient formulation strategies.
This review will provide a brief overview of basic principles involved in bone composition, the impact of dietary nutrients on bone composition, and a critical assessment of methods used to quantify bone integrity in clinical and research settings as presented earlier (Crenshaw et al., 2013). Additional background will be provided to assess the potential for identification of nutrient-induced cellular signals that may provide diagnostic indicators of physiological perturbations that lead to failures in skeletal integrity in swine. Specific cellular signals involved in vitamin D and bone homeostasis have not been clearly elucidated for pigs, which leads to speculative recommendations (Crenshaw et al., 2014). Our recent research interest in vitamin D and skeletal abnormalities was stimulated by an accidental omission of vitamin D from a premix fed to our research herd. Efforts to explain the symptoms induced has led us to explore new pathways recently discovered. A better understanding of the signaling pathways by which vitamin D alters endochondral ossification will enhance development of treatments to prevent lameness and bone related disorders in pigs.
Bone composition and integrity
Diagnosis of clinical symptoms in nutrient based problems. Before discussing the major nutrient induced signals involved in endochondral ossification, a brief overview of bone composition and methods used to diagnose bone will be summarized from a recent review (Crenshaw et al., 2013). This review provided a foundation for use in diagnosis of nutrient-induced skeletal integrity issues in swine.
Bone tissue composition. The water and fat content of bone varies with age, type of bone, and nutrient inputs. Therefore, the mineral content expressed as a percentage of the dry, fat-free weight is a better descriptor of the extent to which the organic matrix has become saturated with mineral (Crenshaw, 2001). On a dry, fat-free basis, approximately 56% of the entire skeleton is ash. The percent ash varies from 62 to 72% in cortical bone from mature sows (Crenshaw et al., 2013) to 44 to 46% in bones from young pigs that are mostly trabecular such as ribs or vertebrae. Thus, selection of a single bone sample and animal age are critical determinants for comparison if percentage ash is used for diagnostic assessment of mineral adequacy. However, over a range in percent ash, the Ca and P content of bone remains constant. The inorganic ash contains Ca (38 to 40%) and P (17 to 19%) in a 2.1:1 ratio. If Ca and P values deviate from these values, then the analytical methods should be questioned. The amount of ash may change in response to diet, but the Ca and P composition of the ash remains constant.
Bone strength. Bone integrity (soundness) is affected by both the organic and inorganic materials that compose the tissue. Bone tissue is a composite material which requires synthesis of an organic matrix by osteoblast cells that are eventually embedded in an extra-cellular matrix. The matrix is composed primarily of collagen fibrils arranged in helical strands with proteoglycan polymers inter-dispersed within the matrix. With time, hydroxyapaptite-like [(Ca3(PO4)2)3·Ca(OH)2] mineral crystals form within the collagen helical matrix. Systemic hormones and localized growth factors stimulate osteoblast proliferation and differentiation with consequences on the rate and accumulation of the organic matrix. However, the actions do not act directly stimulate mineral crystal formation. Attempts to increase mineralization by over-supplementation of diets will down-regulate homeostatic mechanisms and decrease the efficiency of nutrient use.
The combination of the organic matrix and mineral crystals define the material strength properties of bone. The combination of collagen fibers, which contribute primarily tensile (resistance to stretching) properties, and mineral crystals, which contribute primarily compressive (resistance to compression) properties produces an anisotropic material with properties that cannot be explained by the summation of the individual components. Normal loads imposed on a bone are not singularly a tensile or compressive force. Rather, most forces imposed on live animals involve a combination of these two forces to produce a bending load. One surface of the bone is compressed while the other surface is under tension (Crenshaw et al., 1981a). Thus, the combination of materials, collagen and mineral crystals, provide a cumulative response to forces that cannot be explained by a single material.
Selection of a single bone for diagnosis of the animal
Which bone to sample? Decisions on selection of a single bone sample to represent the entire animal should be based on how well the bone reflects changes in the entire animal at the age of sample collection. All bones within the skeleton do not grow at the same rate and thus, do not respond the same to nutrient inputs. Use of dual energy x-ray absorpitometry (DXA) to measure the entire pig and single bones in response to dietary nutrient inputs illustrates this principle. In young pigs (25 to 30 kg) femur ash provided a better fit to dietary P inputs than fibula ash relative to responses in the entire pig (Crenshaw et al., 2009). The fibula tended to overestimate whole body bone mineral content (BMC) at low P intakes, but underestimated BMC at high intakes. As the entire pig, not the femur or fibula, consumed the diet, the bone which reliably predicted the entire pig seemed to be a reasonable choice. However, in a separate study with older pigs (40 to 120 kg) differences among bones (femur, front feet, or hind feet) were not dramatically different in their fit as predictors of whole body BMC (Table 1), but predictions based on fibulas over-estimated the whole body BMC. Thus, selection of limb bones from pigs at market weight (120 kg) are not as critical as the bone selected at younger ages (< 30 kg), consistent with earlier conclusions (Crenshaw et al., 1981b).
Table 1. Regression equations for the use of individual standardized a bone traits as predictors of whole body bone mineral content of pigs (rPBMC) from 40 to 120 kg
Turn-around time and pitfalls in methods to assess mineral status
Methods to assess bone. Traditional methods used to assess skeletal tissue integrity can be broadly classified into 3 approaches (Table 2) which include histology, gravimetric, and mechanical procedures. All 3 procedures, with exception of DXA scans, require a terminal approach and each provide unique, and often different conclusions with regards to nutrient inputs. Each method also has limitations and pitfalls that must be considered in a decision to employ the method or interpret the results for a final diagnosis. Clinical diagnosis requires rapid turn-around to deal with acute issues. Simple, rapid methods, such as visual appraisals, quantifying the incidence of occurrences, and a terminal assessment of the bone ash content are the first-line approaches to deal with acute cases. Long-term, chronic issues can potentially be resolved with additional samples, such as the front foot, collected from marketed animals and submitted for DXA scans to determine mineral content. Use of histology and mechanical tests are more quantitative than measures of bone ash, but require a longer turn-around time for results and are more expensive. Additionally, the measures of bone ash are often only used to diagnosis clinical symptoms already evident in the herd. A method to measure a trait that predicted nutritional adequacy prior to evidence of clinical symptoms would be ideal.
The use of DXA scans of a single bone or foot, a relatively rapid tool for diagnosis, has limitations. First, even given the accuracy of the DXA scans, the results still represent the ash content which is not necessarily a reflection of bone integrity. Second, DXA scans do not identify joint lesions and focal failures which often are predisposing symptoms for lameness.
Table 2. Methods used to assess skeletal tissues in response to nutrient inputs

Inferences for nutritional intervention
Recovery from periods of nutrient deficiencies. A discussion of concepts related to the effects that dietary concentrations of Ca and P have on the accumulation of bone mineral (ash) in pigs is beyond the scope of this review. Deficiencies of these nutrients lead to an under-mineralized bone matrix, deformed limbs (rickets), and spontaneous fractures (mechanical failures). Numerous research papers, reviews and texts have focused on these topics. Guidelines for the amounts of Ca and P supplied and the ratio of Ca to P, especially under conditions of marginal P intake, were relatively well-established until the introduction of phytase supplements as a common feed ingredient. Development of recommendations related to dietary Ca and P supplements in diets that incorporate various phytase products are ongoing. Variant feed formulations based on phytase inclusions may contribute to some of the escalated lameness issues, but quality control issues in feed management, which affect phytase stability, are more likely an issue. However, the focus of this paper is to address guidelines for the assessment of bone to establish if animals have been fed diets within an acceptable safe range of nutrient inputs.
The recent escalation of lameness cases associated with vitamin D has highlighted concerns for quality control issues in diet formulations (Arnold et al., 2014). Failures in quality control procedures for feed management may contribute to delivery of diets with un-intentional deficiencies. The inability of growing pigs to recover skeletal mineral content after a brief period of mineral deficiency was recently reported (Aiyangar et al., 2010). Within 4 wk, a 61.6% reduction in whole body BMC was induced in young pigs fed a diet with 70 vs 150% of Ca and P requirements based on NRC, 1998 guidelines. Whole body DXA scans of the same animals at repeated intervals revealed that BMC was not recovered over a subsequent 8 wk period, even if the pigs were fed diets with 150% of Ca and P requirements. However, femurs collected after the 8-wk recovery period had apparently regained equal strength properties as those from pigs fed control diets throughout the entire trial. Recovery of femur strength, but not whole body BMC can be attributed to either preferential partitioning of mineral reserves to load-bearing bones, potentially at the determent of non-load bearing bones, or to a shift in the distribution of mineral reserves within bone to align the limited resources with the direction of applied loads.
These results further illustrate the discrepancy between assessments based on ash and mechanical test properties. Additional disparate results based on ash vs. mechanical properties have been reported in nursery pigs (Rortvedt et al., 2012), growing-finishing pigs (Iwicki et al., 2011) and to an extent by other researchers (Létourneau-Montminy et al., 2011). Guidelines to identify bone strength properties that reflect acceptable ranges have not been defined. Results from mechanical tests of individual bones have been used to describe animal responses to nutrient inputs. The amount of nutrients required to maximize bone strength exceeds the amount required to maximize bone ash (Crenshaw, 1986; NRC, 1998). Thus, the ash content of bone is not directly proportional to the strength properties of bone. Additionally, procedures used to measure bone mechanical traits are not standardized across laboratories. The time required for mechanical test procedures and requirements of specialized equipment often preclude the routine use of these procedures as a clinical diagnostic method. Thus, bone mechanical tests are not recommended for use in clinical assessment of lameness cases, rather such test methods remain as a technique for assessment of hypothesis-driven research projects.
Lessons learned from hump-back pigs. Prior to 2008, we understood that kyphosis was an idiopathic disease which occurred sporadically in swine and produced hump-back pigs with spinal deformities. Although kyphosis was problematic in afflicted herds, the disorder was considered of little consequence to the overall industry. After a flare-up in our research herd in 2008, we have been able to induce kyphosis under controlled conditions by use of diet manipulation. The efforts to characterize the disorder have led to additional insights in bone and connective tissue development that may link lesions of mineralization and osteochondrosis (OC).
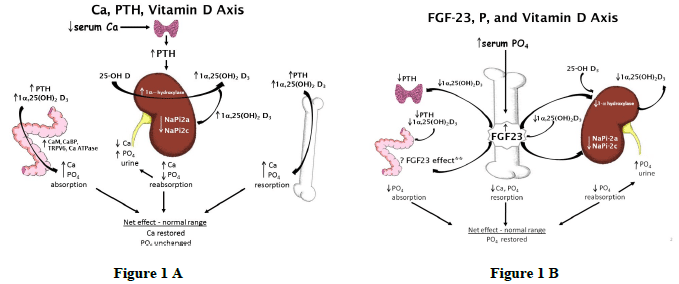
Although vitamin D has traditionally been indirectly associated with bone development through regulation of Ca homeostasis, a newly discovered hormone has provided additional insights into vitamin D and P homeostasis which involves bone. Traditional pathways for vitamin D regulation of Ca and P homeostasis (Figure 1A) have focused on regulation of vitamin D by parathyroid hormone (PTH) in response to fluctuations in serum Ca concentrations. The hormones induce changes in target tissues of the kidney, GI tract, and bone. More recently, evidence for a direct role of vitamin D in bone development was provided through identification of the vitamin D receptor (VDR) in bone cells and by new insights in cellular signal pathways that control P homeostasis. The newly discovered phosphaturic hormone, fibroblast growth factor 23 (FGF23) has been characterized (Lanske et al., 2014; Martin et al., 2011; and Sitara et al., 2006). FGF23 is primarily produced in the bone osteocyte. FGF23 is responsible for P homeostasis through a pathway that involves feedback regulation between FGF23, vitamin D, and P (Figure 1B).
Figure 1A and 1B. Comparison of the traditional Ca, PTH, and vitamin D axis to describe Ca homeostasis in response to a reduction in serum Ca with the novel FGF23, P, and vitamin D axis in response to an increase in serum P. (From Crenshaw et al., 2011)
The novel pathway for FGF23, vitamin D, and P homeostasis now offers opportunities to improve P efficiency without compromising skeletal growth and animal well-being. Historically swine diets were fortified with excess Ca and P with minor incentives to improve nutrient efficiency. Constraints on dietary supplemental P are now driven by ingredient costs and environmental concerns. Thus P, not Ca, is typically more limited in swine diets (Crenshaw 2001). New concepts revealed by the FGF23 pathway have challenged the traditional axiom and disclosed signals and feedback inhibition among P, vitamin D metabolites, and PTH with a critical regulatory component attributed to FGF23. A central component that linked FGF23 with P homeostasis and renal function involved identification of bone as the primary tissue for FGF23 synthesis, in essence ascribing an endocrine gland function to bone. By identifying the nutritionally induced responses in the novel pathway for regulation of P homeostasis, applied feeding strategies can be developed to improve efficiency of dietary P use and vitamin D homeostasis in swine.
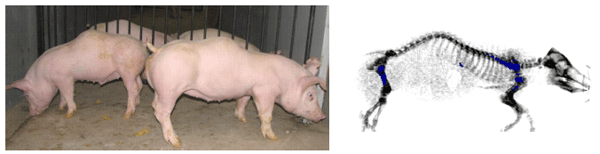
As mentioned previously, a challenge to explore the alternate pathway for vitamin D-mediated P homeostasis was stimulated, in part, by an outbreak and subsidence of kyphosis (~20% incidence) in pigs produced by the UW Swine Research and Teaching Center (SRTC) herd during a 4 month period in 2008 (Figure 2). The outbreak and subsidence of kyphosis in the closely monitored research herd occurred with no changes in animal genetics or health status. In a survey of pigs from 3 herds with a high incidence of kyphosis (Nielsen et al., 2005), lesions predominated in the 14th, 15th, and 16th thoracic vertebra. The wedge-shaped vertebra, characteristic of kyphosis, displayed histological lesions characterized as a failure of endochondral ossification. The FGF peptides are implicated in endochondral development (Horton and Degnin, 2009). Gross anatomical skeletal features of FGF23-null mice (Sitara et al., 2006) are reminiscent of skeletal deformities displayed in pigs with kyphosis.
The kyphosis outbreak at SRTC stimulated a series of trials that eventually linked the disorder to an accidental omission of vitamin D3 from a custom-mixed vitamin premix that was fed to the entire herd for 4 months. The symptoms were initially observed in growing pigs at SRTC fed diets with marginal amounts of Ca and P. However, the outbreak and subsidence of kyphosis in these pigs coincided with the period during which the deficient premix was fed to the gestating sows that produced the pigs with kyphosis.
Figure 2. Young pigs (~ 30 kg BW) displaying kyphosis and a DXA scan image of a ~ 20 kg pig to show abnormal spinal column curvature. (From Rortvedt and Crenshaw, 2012).
A controlled study was subsequently conducted to confirm the inferences of a maternal diet effect on vitamin D induced kyphosis in young pigs. The objective was to determine if sows fed a gestation diet without supplemental vitamin D produced pigs that displayed kyphosis. The weaned pigs were fed diets without supplemental vitamin D, and either adequate (120% of requirements, NRC 1998) or marginal (80% of requirements, NRC 1998) amounts of Ca and P. Young pigs fed marginal nursery diets and produced by sows fed a gestation diet with no supplemental vitamin D developed kyphosis (21% incidence) and displayed reduced growth and skeletal mineral content determined from DXA scans by 9 wk of age (Rortvedt and Crenshaw, 2012). At 9 wk, none of the pigs from sows fed diets supplemented with vitamin D (280 IU D3/kg) displayed evidence of kyphosis. However, by 13 wk, evidence of kyphosis (25% incidence) was detected in pigs fed marginal diets even if the pigs were produced by sows fed diets with supplemental vitamin D. At 13 wk the kyphosis incidence in pigs from sows fed diets with no supplemental vitamin D had increased to 33%. The effects of vitamin D concentrations in maternal diets affected the time required for pigs to display symptoms of kyphosis. The time effect was consistent with an involvement of maternal diets in predisposing pigs to skeletal defects.
Based on earlier research (Aiyangar et al., 2010; Crenshaw, 1986; NRC, 1998), the marginal dietary Ca and P concentrations used to induce kyphosis in the above experiment were not dramatic deficiencies, but may have been exacerbated by lack of supplemental vitamin D. The kyphosis responses were not expected. Either marginal deficiencies of all 3 nutrients exacerbated responses or the life-cycle phases in which the deficiencies were imposed were critical. As discussed in subsequent sections, the vitamin D status of the sows may have contributed to the observed responses.
Maternal carry-over effects. Evidence to support maternal carry-over effects of dietary vitamin D on subsequent pig responses were recently reported (Rortvedt and Crenshaw, 2012; Rortvedt-Amundson and Crenshaw, 2013). Whole body bone mineral density (BMD) was reduced (~20%) in pigs produced by gilts fed 0 or 325 IU D3/kg and fed diets with no supplemental vitamin D and 120% recommended P requirements. However, BMD was not reduced in pigs fed the same nursery diets if they were produced by gilts fed 1,750 IU D3/kg during gestation.
In the same experiment differences due to maternal diet effects in whole body BMD were not detected in pigs at birth and weaning. However, maternal diet effects were detected in mRNA expression of genes involved in vitamin D metabolism in kidney and intestine of the neonatal pigs (Rortvedt-Amundson and Crenshaw, 2014). Maternal diets with excess dietary vitamin D increased mRNA expression of 24,25 hydroxylase (CYP24A1) in pigs at weaning, implying an increased ability of the pigs to degrade vitamin D.
The effects due to maternal diets in this experiment and an earlier experiment (Rortvedt et al. 2011) have identified apparent deficiencies that were induced in relatively short time periods even in pigs produced by sows fed the amounts of vitamin D routinely fed to the SRTC breeding herd. Updated estimates of vitamin D requirements (NRC, 2012) imply that the vitamin D supplements in the SRTC diets are not sufficient.
Collectively, these results are consistent with maternal effects of hypovitaminosis D induced bone abnormalities in the fetal pig. The induction of abnormalities are possibly initiated in utero in sows fed diets with marginal or deficient vitamin D concentrations. The abnormalities are detectable at birth and weaning if assessments are based on molecular signals, but the gross abnormalities of bone tissues are not evident until the pigs are nutritionally stressed by marginal Ca and P after weaning. Thus, the measurement of both gross and molecular characteristics in the pig at multiple developmental stages is necessary to elucidate the critical regulatory signals involved in the initiation of lesions induced in the swine hypovitaminosis D kyphosis model.
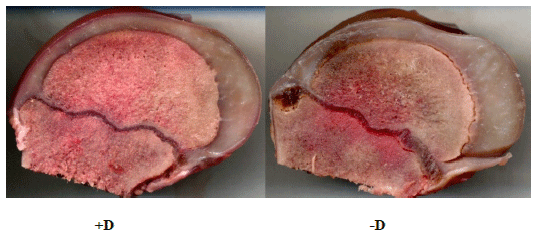
Kyphosis - lesions of endochondral ossification. Gross observations of spines from pigs with kyphosis revealed abnormalities of the vertebral growth plate with evidence of retained cartilage, similar to abnormalities reported in pigs with lesions of OC (McCoy et al., 2013; Ytrehus et al., 2007). Other gross abnormalities were not initially observed in live animals, but further analysis of femurs collected from these pigs revealed growth plate abnormalities, which included focal regions of irregular physeal widths (Figure 3). These observations support the need to further evaluate changes in molecular signals that are involved in endochondral ossification, especially signals that may be altered by insufficient dietary vitamin D during fetal and early neonatal development.
Figure 3. Sections of the distal femur to illustrate irregular physeal widths. Sections were collected from 9 wk pig fed +D diets (+D) or diets with no supplemental vitamin D (-D) for 5 wk. (Unreported results).
The femur and vertebrae are both formed by endochondral ossification, a process in which calcified cartilage is coupled with replacement by calcified bone during normal bone formation. An un-coupling of this process leads to an abnormal, wider growth plate, including irregular widths and retained, excess cartilage. The modeling and remodeling of the extracellular matrix (ECM) is the rate-limiting step in endochondral ossification (Ortega et al., 2004; Stickens et al., 2004). Critical proteins and signals required for normal endochondral ossification include the family of matrix metalloproteinases (MMP), particularly MMP9, a gelatinase, and MMP13, a collagenase, and vascular endothelial growth factor (VEGF) (Ortega et al., 2004). All 3 signals are directly or indirectly mediated by vitamin D. Evidence to directly link expression of these signals with vitamin D status is not available for pigs.
Lameness research in pigs and other production animals has typically focused on characterizing the later stages of lesions rather than the initiation of lesion development. The swine hypovitaminosis D kyphosis model provides a means to study cellular signals involved in the initiation of growth plate abnormalities as the model provides a method to predictably induce a spontaneous lesion. Characterizing the dynamics of target cells and the cellular signals which are disrupted during abnormal spinal column and femur development of neonatal pigs will potentially lead to specific and effective interventions for prevention of bone abnormalities.
We are continuing to learn from the humpback pig, which has helped us to focus on the pivotal signals that need to be measured. In the past, swine lameness was broadly grouped into either problems associated with mineralization or problems produced by lesions of OC. Experiments to understand the kyphosis incidence in our herd has led to potential signaling pathways that may link kyphosis and OC.
Pivotal signals regulated by nutrient inputs that affect endochondral ossification
Bio-markers for bone. The use of numerous bio-markers to identify bone integrity traits have been attempted, but at best these bio-markers have only proven to be qualitative and of limited value in diagnosis of skeletal lameness. As a result of homeostasis, serum concentrations of nutrients such as Ca and P and even 25-OH vitamin D are of limited value for assessments of skeletal integrity concerns, even though these nutrients are the primary dietary factors that drive skeletal mineral accumulation. A better understanding of the pivotal nutrient-induced cellular signals that affect bone may provide potential use for improvement of methods for rapid diagnosis of swine lameness problems.The pivotal signals that may reflect nutritional and immunological inputs appear to be FGF23, RANKL/OPG, CYP27B1, CYP24A1, VEGF, and MMP.
As discussed previously, FGF23 is involved in vitamin D and P homeostasis. FGF23 is produced by osteoblast and osteocyte cells and is regulated by vitamin D and P (Sitara et al., 2006; Crenshaw et al., 2011; and Lanske et al., 2014). Active vitamin D increases FGF23 production in bone which then acts systemically to up-regulate renal Na-P transporters to increase P reabsorption, thus decreasing circulating P. FGF23 downregulates 1α-hydroxylase and reduces activation of vitamin D (Figure 1b). Given the expression of the VDR in chondrocytes, vitamin D potentially has a direct effect on bone tissue production of FGF23. In chondrocyte specific VDR knockout mice, FGF23 release was increased by vitamin D through a chondrocyte-mediated signaling pathway (Masuyama et al., 2006; St-Arnaud, 2008). The signaling factor released from chondrocytes has not yet been elucidated. Our recent data (Rortvedt- Amundson and Crenshaw 2014), revealed an increase in bone FGF23 mRNA expression in nursery pigs fed supplemental D, especially if pigs were fed diets with excess P. The newly identified roles of FGF23 suggest an endocrine role of bone tissue in P homeostasis, beyond that of merely being a storage site for Ca and P (Masuyama et al., 2006; St-Arnaud, 2008).
Excess release of FGF23 may down-regulate activation of vitamin D and induce an apparent vitamin D deficiency, even though vitamin D is supplemented in the diet. This scenario is more likely under conditions of excess dietary P. Swine diets would not typically be expected to contain excess P, but adoption of the use of phytase enzymes to release more P from plant ingredients and the inclusion of ethanol co-products, which contain excess P, has increased the potential for swine diets to contain excess P. Production of excess FGF23 may reduce the efficiency of P use and lead to an oversupplementation of a mineral that contributes to surface water pollution.
To our knowledge, FGF23 gene expression, nor the protein has been successfully measured in the pig until our efforts reported at this meeting (Rortvedt-Amundson and Crenshaw, 2014). The data reported on bone tissue FGF23 mRNA expression via qPCR assays were altered by nutrient inputs, but contained considerable variation. More work is needed to support the role of FGF23 as a pivotal signal in swine. We are more confident in our qPCR results to measure mRNA expression of RANKL, CYP27B1, and CYP24A1 in pig tissues. Measures of VEGF, and MMP have not yet been attempted.
Two main types of cells are involved in bone formation, the osteoblast (Obl) and the mature osteocyte. One cell type is involved in bone resorption is the osteoclast (Ocl). Bone formation and resorption processes are typically coupled in remodeling. Thus, no net accumulation or loss occurs in adult animals at maintenance, but homeostatic mechanisms can alter the balance. In growing animals, formation exceeds resorption, so bone mineral accumulates. The Ocl are involved in bone resorption. These cells do not have receptors for systemic hormones such as PTH or vitamin D, but are controlled via the Obl lineage of cells. Recent research has focused on the signals and cells that produce the signals to induce Ocl recruitment and activation of resorption at specific locations. Cells of the Obl lineage complete various functions to release signals (RANKL and OPG) in response to systemic hormones.
Support for direct roles of 1,25 OH D in bone formation are based on the presence of the VDR in both chondrocytes and Obl. Most systemic roles of 1,25 D are mediated via mineral homeostasis, however under conditions of rescue diets in VDR-null mice, bone mineralization was restored but growth plate deformities were detected (Li et al, 2002; St-Arnaud, 2008) supporting a direct role for vitamin D in endochondral bone formation. In proliferative and hypertrophic chondrocytes, 1,25 OH D induces the synthesis of an unknown protein that increases FGF23 release and increases the synthesis of RANKL and VEGF. RANKL and VEGF act to stimulate Ocl activation and recruitment to resorb the hypertrophic zone, thus. maintaining a normal chondro-osseous junction and vascular invasion of the calcified hypertrophic zone. This process of endochondral ossification allows bone growth and a normal growth plate width. Disruption of this process results in retention of cartilage in focal regions and development of lesions such as OC.
Links between bone and the immune system. Lameness cases in swine herds are often confounded with herd health status. Our research, to date, has involved pigs from a herd with no major health challenges. Links have been implied between bone lesions and immune function, but direct mechanisms are difficult to establish. Both Ocl and macrophages are derived from the same progenitor hematopoietic stem cells and respond to many of the same signaling pathways. The role for vitamin D in immune response and the extra-renal activation of 25OH to 1,25 OH D as the primary modulator of vitamin D responses are controversial. Treatment with 1,25 OH D has been used to suppress several autoimmune disorders such as rheumatoid arthritis, type 1 diabetes, and multiple sclerosis (Deluca, 2014). Clearly more research is needed to establish the pivotal signals to link bone abnormalities and health challenges.
Conclusions
Clinical cases that involve lameness issues in pigs often lead to questions about the adequacy of nutrient formulations, especially dietary Ca and P, and more recently vitamin D. Attempts to correct the increased incidence of lameness by over-supplementation of nutrients most often does not resolve the problems and may contribute to further complications. Over-supplementation of nutrients, particularly Ca and P, does not necessarily allow pigs to recover from skeletal integrity challenges that may have been imposed by brief periods of deficiency. Accurate diagnosis of nutrient deficiencies require standardized sampling and analysis procedures. Use of DXA technologies offer accurate and rapid turn-around for specimen analysis of BMC, but simply provide information on the ash content of the skeleton or individual bones.
A better understanding of the cellular signals that control endochondral ossification will lead to methods that are better predictors pf the alterations that induced the lesions. The hypovitaminosis D kyphotic pig model provides an opportunity to characterize the signals involved in the initial stages of lesions in the abnormal growth plates.
References
Aiyangar, A. K. T. D. Crenshaw, A. G. Au, H. L. Ploeg. 2010. Recovery of bone strength in young pigs from an induced short-term dietary calcium deficit followed by a calcium replete diet. Medical Engineering & Physics 32:1116-1123.
Arnold, J., D. M. Madson, S. M. Ensley, J. P. Goff, C. Sparks, G.W. Stevenson, T. D. Crenshaw, C. Wang, and R. L. Horst. 2014. Survey of vitamin D concentrations in swine serum across different stages of production and an evaluation of supplemental vitamin D stability in premixes used in swine diets. J. Swine Health Prod. (In review)
Crenshaw, T. D., E. R. Peo, Jr., A. J. Lewis and B. D. Moser. 1981a. Bone strength as a parameter for assessing mineralization in swine: A critical review of techniques involved. J. Anim. Sci. 53:827-835.
Crenshaw, T. D., E. R. Peo, Jr., A. J. Lewis, B.D. Moser and D. Olson. 1981b. Influence of age, sex, and calcium and phosphorus levels on the mechanical properties of various bones in swine. J. Anim. Sci. 52:1319-1329.
Crenshaw, T. D. 1986. Reliability of dietary Ca and P levels and bone mineral content as predictors of bone mechanical properties at various time periods in growing swine. J. Nutr. 116:2155-2170.
Crenshaw, T. D. 2001. Calcium, Phosphorus, Vitamin D, and Vitamin K in Swine Nutrition. In: Swine Nutrition. A. J. Lewis and L. L. Southern eds. CRC Press. p 187-212.
Crenshaw, T. D., J. R. Danielson, L. E. Hoffman, and D. K. Schneider. 2009. Femurs are more accurate than fibulas as predictors of whole body bone mineral content in growing pigs. J. Anim Sci 87:(E-Suppl 2) 510.
Crenshaw, T. D., L. A. Rortvedt, Z. Hassen. 2011. A novel pathway for vitamin D-mediated phosphate homeostasis: Implications for skeletal growth and mineralization. J Anim Sci 89(7):1957-1964.
Crenshaw,T.D., D.K. Schneider, C.S. Carlson, J. B. Parker, J.P. Sonderman, T.L. Ward, M.E. Wilson. 2013. Mineral concentrations in tissues and lesions of osteochondrosis in bones collected from prolific sows across parities 0 through 7. J. Anim. Sci. 91:1255–1269.
Crenshaw, T. D., J. L. Reichert, J. R. Booth, D. K. Schneider, and L. A. Rortvedt-Amundson. 2013. Clinical diagnosis of skeletal integrity in swine. Leman Swine Veterinary Conference Proceedings. September 17, 2013. St Paul MN.
Crenshaw, T. D., L. A. Rortvedt-Amundson, J. A. Cuaron, J. R. Bergstrom, and G. Litta. 2014. TRIENNIAL GROWTH SYMPOSIUM: Vitamin D - Establishing the basics to dispel the hype. J. Anim. Sci. 92:883-886.
Deluca, H. F. 2014. TRIENNIAL GROWTH SYMPOSIUM: Vitamin D: Bones and beyond. J. Anim. Sci. 92:917- 929.
Horton, W. A., and C. R. Degnin. 2009. FGFs in endochondral skeletal development. Trends in Endocrinology and Metabolism. 20(7): 341-348.
Iwicki, L. A., J. L. Reichert, J. R. Booth, D. K. Schneider, and T. D. Crenshaw. 2011. Recovery of bone mineralization and strength after a marginal dietary calcium deficiency in growing pigs. J. Anim. Sci. 89 (e-Suppl. 1): 585.
Lanske, B., M. J. Densmore, and R. G. Erben. 2014. Vitamin D endocrine system and osteocytes. BoneKEy Rpt 3:494. (doi:10.1038/bonekey.2013.228).
Létourneau-Montminy, M.P., Lovatto, P.A. and Pomar, C. 2011. Effect of phosphorus and calcium depletionrepletion periods on the digestive and metabolic utilization of dietary phosphorus and calcium in growing pigs. J Anim. Sci. Vol. 89, E-Suppl. 3, 180.
Lin, R., N. Amizuka, T. Sasaki, M. M. Aarts, H. Ozawa, D. Goltzman, J. E. Henderson and J. H. White. 2002. 1α,25-dihydroxyvitamin D-3 promotes vascularization of the chondroosseous junction by stimulating expression of vascular endothelial growth factor and matrix metalloproteinase 9. J Bone Miner Res. 17(9):1604-1612.
Madson, D. M., S. M. Ensley, P. C. Gauger, K. J. Schwartz, G. W. Stevenson, V. L. Cooper, B. H. Janke, E. R. Burrough, J.P. Goff, and R. L. Horst. 2012. Rickets: case series and diagnostic review of hypovitaminosis D in swine. J. Vet. Diag. Invest. 24:1137-1144.
Martin, A., S. Liu, V. David, H. Li, A. Karydis, J. Q. Feng, L. D. Quarles,. 2011. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor FGF23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB. 25:2551-2562.
Masuyama, R., I. Stockmans, S. Torrekens, R. Van Looveren, C. Maes, P. Carmeliet, R. Bouillon and G. Carmeliet. 2006. Vitamin D receptor in chondrocytes promotes osteoclastogenesis and regulates FGF23 production in osteoblasts. J Clin Invest. 116(12):3150-3159.
McCoy, A. M., F. Toth, N. I. Dolvik, S. Ekman, J. Ellermann, K. Olstad, B. Ytrehus and C. S. Carlson. 2013. Articular osteochondrosis: a comparison of naturally-occurring human and animal disease. Osteoarth Cartil. 21:1638-1647.
Nielsen, L. W. D., P. Hogedal, J. Arnbjerg, and H. E. Jensen. 2005. Juvenile kyphosis in pigs - A spontaneous model of Scheuermann's kyphosis. APMIS. 113(10): 702-707.
NRC. 1998. Nutrient requirements of swine. 10th revised edition. National Academy Press. Washington,DC.
NRC. 2012. Nutrient requirements of swine. 11th revised edition. National Academy Press. Washington,DC.
Ortega, N., D. J. Behonick and Z. Werb. 2004. Matrix remodeling during endochondral ossification. Trends Cell Biol. 14(2):86-93.
Rortvedt, L. A., Z. Hassen, T. D. Crenshaw. 2011. Growth, DXA skeletal traits, and spinal curvature are compromised within four weeks in pigs fed diets with no supplemental vitamin D. J. Anim. Sci. 90(e-Suppl. 2):1.
Rortvedt, L.A. and T.D. Crenshaw. 2012. Expression of kyphosis in young pigs is altered by vitamin D supplementation of maternal diets. J Anim Sci. 90:4905-4915.
Rortvedt, L. A., D. K. Schneider, and T. D. Crenshaw. 2012. Bone ash and strength traits of young pigs fed diets with no supplemental vitamin D were compromised within a four-week trial. J. Anim. Sci. 90 (e-Suppl. 3):564.
Rortvedt-Amundson, L. A. and T. D. Crenshaw. 2013. Pig bone trait responses to maternal vitamin D intake depend on nursery diet vitamin D and P concentrations. J Anim Sci. 91 (e-Suppl. 2):110.
Rortvedt-Amundson, L. A. and T. D. Crenshaw. 2014. Maternal and nursery dietary vitamin D concentrations altered tissue mRNA expression. IPVS Congress (accepted).
Sitara, D., M. S. Razzaque, R. St-Arnaud, W. Huang, T. Taguchi, R. G. Erben, B. Lanske. 2006. Genetic ablation of vitamin D activation pathway reverses biochemical and skeletal anomalies in Fgf-23-null animals. Am J Pathol. 169:2161-2170.
St-Arnaud, R. 2008. The direct role of vitamin D on bone homeostasis. Arch Biochem Biophys. 473(2):225-230.
Stickens, D., D. J. Behonick, N. Ortega, B. Heyer, B. Hartenstein, Y. Yu, A. J. Fosang, M. Schorpp-Kistner, P. Angel and Z. Werb. 2004. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development. 131(23):5883-5895.
Ytrehus B., Carlson C. S., Ekman S. 2007. Etiology and pathogenesis of osteochondrosis. Vet Pathol. 44:429-448.
Content from the event:
Related topics:
Authors:

Recommend
Comment
Share
Recommend
Reply

Would you like to discuss another topic? Create a new post to engage with experts in the community.