Use of an essential oil blend formulation (EOBF) as an effective disinfectant against pathogenic luminescent Vibrio bacteria
Author details:
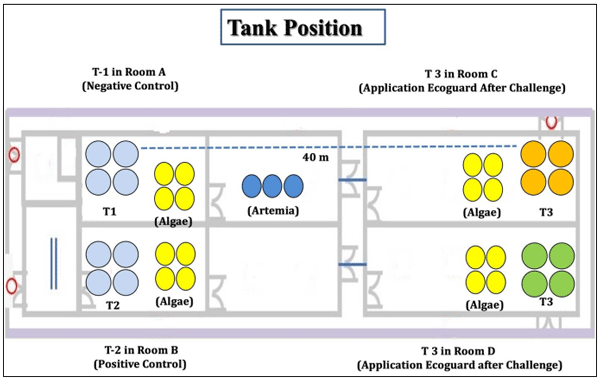
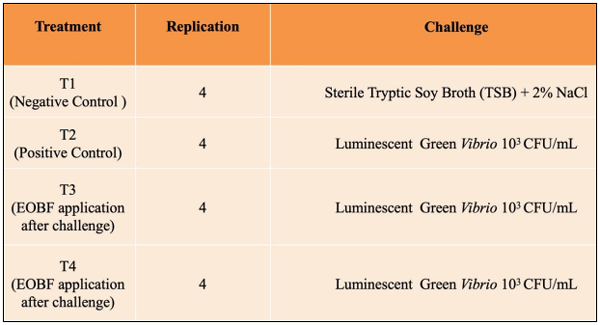
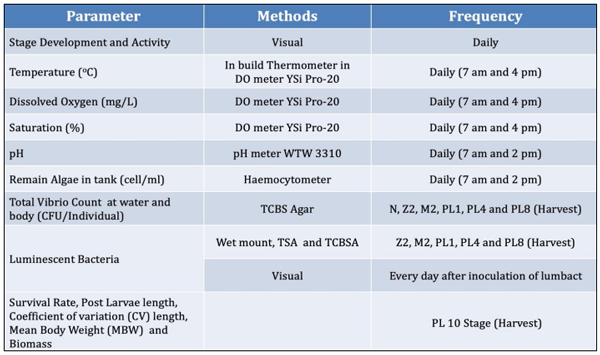
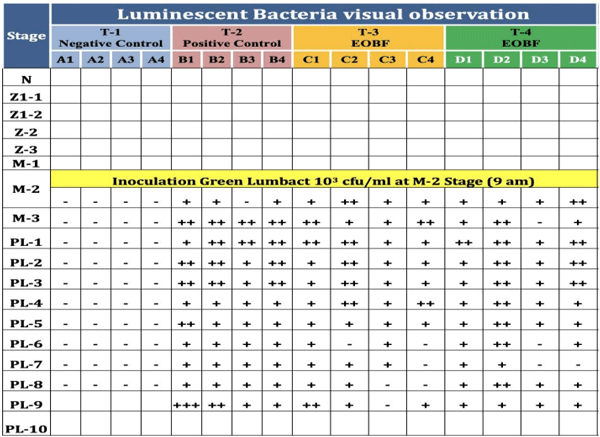
Vibrio, mainly Vibrio harveyi, V. campbellii, V. parahaemolyticus and V. splendidus, especially in their luminescent form are considered highly pathogenic to shrimp larvae. An effort was made to minimize the luminescent Vibrio load using an essential oil blend formulation (EOBF) consisting of Eucalyptus oil, jasmine oil, and gardenia oil in equal proportions. An eighteen-day small hatchery scale trial was initiated, starting from the nauplii stage. The shrimp were distributed into three groups and four replicates each: a negative control, positive control with four replicates, and an EOBF treatment group. The shrimp at the mysis stage were challenged using a sublethal dose of 103 CFU/mL luminescent Vibrio harveyi.
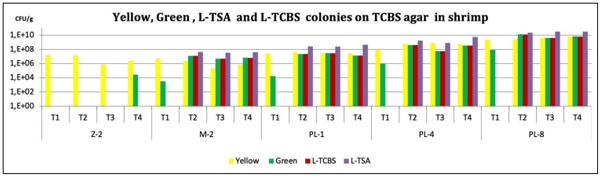
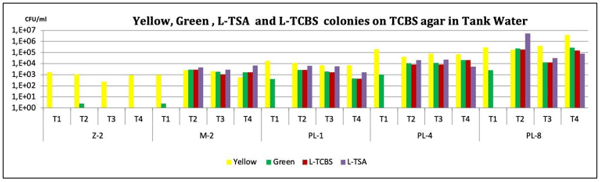
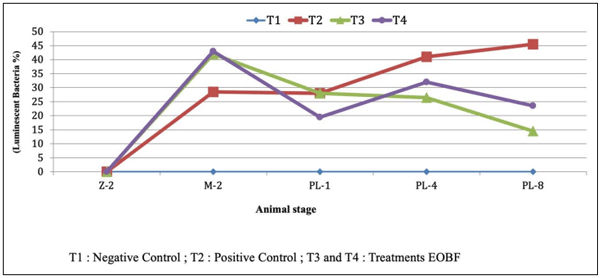
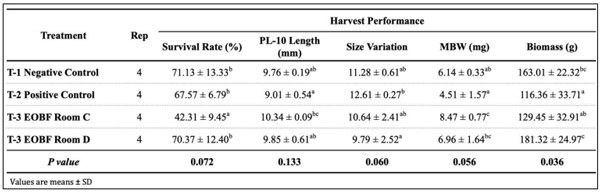
The obtained results indicated that the presence of harmful Vibrio in tank water, was almost one log lower in the EOBF group than in the positive control. The presence of pathogenic luminescent Vibrio in the positive control was 45%, whereas it was 19% in the treatment group. In addition, the EOBF-treated had better performance, and productivity. The results of this trial suggest that EOBF can reduce pathogenic Vibrio in hatchery environments and can increase productivity.
Keywords: Pathogenic Vibrio, Luminescent Vibrio, Vibrio parahaemolyticus, Vibrio harveyi, Essential Oil Blend Formulation, Disinfectant. Shrimp hatchery, Penaeus vannamei.
1. Lavilla-Pitogo CR, Albright LJ, Paner MG, Sumaz NA. Studies on the source of luminescent Vibrio harveyi in Penaeus monodon hatcheries. In: Diseases in Asian Aquaculture I. Shariff, M., Subasinghe, R.P. and Arthur J.R. (eds). Asian Fisheries Society, Philippines, 1992, 157-164.
2. Gomez-Gil B, Herrera-Vega MA, Abreu-Grobois FA, Roque A. Bioencapsulation of two different Vibrio species in nauplii of the brine shrimp (Artemia franciscana). Appl. Environ. Microbiol. 1998;64:2318- 2322.
3. Lavilla–Pitogo CR, Leano EM, Paner MG. Mortalities of pond cultured juvenile shrimp Penaeus monodon, associated with dominance of luminescent Vibriosis in the rearing environment. Aquacult. 1998;164:337-349.
4. Nackerdien ZE, Keynan A, Bassler BL, Lederberg J, Thaler DS. Quorum Sensing Influences Vibrio harveyi Growth Rates in a Manner Not Fully Accounted For by the Marker Effect of Bioluminescence. PLoS ONE. 2008;3(2):e1671. https://doi.org/10.1371/journal.pone.0001671
5. Nash G, Charetana N, Cholada T, Anutra A, Phusit P, Pongcham R. Vibriosis and its control in pond-reared Penaeus monodon in Thailand. In: Diseases in Asian Aquaculture I. Shariff M., Subasinghe, R.P. and Arthur J.R. (eds). Asian Fisheries Society. Philippines, 1992, 143-155.
6. Lightner DV. Diseases of cultured penaeid shrimp and prawns. In: Disease diagnosis and control in North American marine aquaculture. Second edition. C.J Sinderman and D.V. Lightner, (eds). Elsevier, Amsterdom, 1988, 8-127 .
7. Karunasagar I, Otta SK, Karunasagar I, Joshna K. Successful Management of white spot disease in shrimp using immunostimulants. Fish Chimes. 1996; 161: 49-50.
8. Mandabi A, Ganin H, Meijler MM. Synergistic activation of quorum sensing in Vibrio harveyi. Bioorg Med Chem Lett. 2015;25:3966-3969.
9. McRose DL, Baars O, Seyedsayamdost MR, Morel FM. Quorum sensing and iron regulate a two-for-one siderophore gene cluster in Vibrio harveyi. P Natl Acad Sci. 2018;115(29):7581-7586.
10. Dobre AA, Gagiu V, Petru N. Anti-bacterial activity of Essential oils against food-borne bacteria evaluated by two preliminary methods. Romanian Biotechnology letters. 2011;16(6):119-125.
11. Falahati M, Tabrizib NO, Jahaniani F. Anti-dermatophyte activities of Eucalyptus camaldulensis in comparison with Griseofulvin. Iran J Pharmacol Ther. 2005;4:80-83.
12. Bachheti RK, Joshi A, Singh A. Oil Content variation and Antimicrobial activity of Eucalyptus leaves oils of three different Species of Dehradun, Uttarakhand, India. International Journal of ChemTech Research. 2011;3(2):625-628.
13. Chowdhury A, Azam S, Jainul MA, Faruq KO, Islam A. Antibacterial Activities and In Vitro Anti-Inflammatory (Membrane Stability) Properties of Methanolic Extracts of Gardenia coronaria Leaves. Int J Microbiol. 2014: 410935. Published online 2014 Feb 19. doi: 10.1155/2014/410935. PMCID: PMC3948643.
14. Yusuf MB, Shaban NZ, Elrashidy FH, Ghaeeb DA. Antioxidant, Anti-inflammatory, Antiproliferative and Antimicrobial Activities of Combretum glutinosum and Gardenia aqualla Extracts in vitro. Free Radicals and Antioxidants. 2020;9(2):66-72. DOI: 10.5530/fra.2019.2.12.
15. Baswa M, Rath CC, Dash SK, Mishra RK. Antibacterial activity of Karanj (Pongamia pinnata) and Neem (Azadirachta indica) seed oil: A preliminary report. Microbios. 2001;105:183–9.
16. Rath CC, Devi S, Dash SK, Mishra RK. Antibacterial Potential Assessment of Jasmine Essential Oil Against E. Coli. Indian J Pharm Sci. 2008;70(2):238–241. doi: 10.4103/0250-474X.41465.
17. Santhanam J, Abd Ghani FN, Basri DF. Antifungal activity of Jasminum sambac against Malassezia sp. and Non-Malassezia sp. isolated from human skin samples. J Mycol. Article. 2014. ID 359630, http://dx.doi.org/10.1155/2014/359630.
18. Zhang XH, Lin H, Wang XL, Austin B. Significance of Vibrio species in the marine organic carbon cycle—A review. Science China Earth Sciences. 2018;61(10). DOI: 10.1007/s11430-017-9229-x.
19. Wright AC, Goodrich-Schenider R, Hubbard MA, Schneider KR. Preventing Foodborne and Non-foodborne Illness: Vibrio parahaemolyticus. FSHN09-01. Gainesville: University of Florida Institute of Food and Agricultural Sciences, 2009. Retrieved June 5, 2015, from http://edis.ifas.ufl.edu/fs146.
20. Timenetsky J, Yanaguita RM, Silva LA. Avaliação de desinfetantes químicos de uso doméstico contra Vibrio cholerae EL TOR (amostra não toxigênica) [Evaluation of household chemical disinfectants for Vibrio cholerae EL TOR (non toxigenic strain)]. Rev Saude Publica. 1992;26(5):328-31. Portuguese. doi: 10.1590/s0034- 89101992000500005. PMID: 1342521.
21. Jeffrey DJ. Chemicals used as disinfectants: active ingredients and enhancing additives. Revue scientifique et technique. PMID: 7548972 Review, 1995.
22. Chen Z, Yu ZH, Fang Y, Xue ZZ. [Experiment observations of the germicidal effects of disinfectants on Vibro cholerae of El Tor biotype in different water bodies]. PMID: 11769638 Chinese, 2001.
23. Newman SG. Disinfectant use in aquaculture 2006. From: https://www.aquaculturealliance.org/advocate/disinfectan t-use-in aquaculture/?headlessPrint=AAAAAPIA9c8r7gs82oWZ BA.
24. Hasan A. Revisiting Vibriosis in shrimp aquaculture. Triggers and co-infections, symptoms and prevention measures for the opportunistic and ubiquitous Vibrio spp. Aquaculture Asia Pacific, 2020. from https://aquaasiapac.com/2020/09/29/revisiting-Vibriosisin-shrimp-aquaculture/.
25. Ching CA, Portal J, Salinas A. Low-salinity culture water controls Vibrios in shrimp postlarvae, 2014. From: https://www.globalseafood.org/advocate/low-salinityculture-water-controls-Vibrios-in-shrimp-postlarvae/.
26. Mandal S, Mandal M. VIBRIO | Vibrio choleraein Encyclopedia of Food Microbiology (Second Edition), 2014. In Thiosulfate-Citrate-Bile Salts-Sucrose Agar From: Principles of Bacterial Pathogenesis, 2001.
27. Cecília de Souza V Wan, Alex HL. Vibrio and major commercially important Vibriosis diseases in decapod crustaceans. Journal of Invertebrate Pathology. 2021;181:107527. https://doi.org/10.1016/j.jip.2020.107527.(https://www.sc iencedirect.com/science/article/pii/S0022201120302330).
28. Prayitno B, Latchford JW. Experimental infections of crustaceans with luminous bacteria related to Photobacterium and Vibrio. Effect of salinity and pH on infectiosity, Aquaculture. 1995;132:105-112. https://doi.org/10.1016/0044-8486(94)00374-W. (https://www.sciencedirect.com/science/article/pii/00448 4869400374W).
29. Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T. 2015;40(4):277-83. PMID: 25859123; PMCID: PMC4378521.
30. Puvaca N, Stanacev V, Glamocic D, Levic J, Peric L, Stanacev V, Milic D. Beneficial effects of Phytoadditives in broiler nutrition. World’s Poultry Science Journal. 2013;69(1):27-34. doi:10.1017/S004393391300003.
31. Friedman M, Henika PR, Mandrell RE. Bactericidal Activities of Plant Essential Oils and Some of Their Isolated Constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica . Journal of Food Protection. 2002;65(10):1545- 1560.
32. Faleiro ML. The Mode of Antibacterial Action of Essential Oils. In: Mendez-Vilas, A., Ed., Science against Microbial Pathogens: Communicating Current Research and Technological Advances, Formatex Research Center, Badajoz, 2011, 1143-1156.
33. Yap PS, Yiap BC, Ping HC, Lim SH. Essential oils, a new horizon in combating bacterial antibiotic resistance. The open microbiology journal. 2014;8:6-14. https://doi.org/10.2174/1874285801408010006.
34. Ultee A, Bennik MH, Moezelaar R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Applied and environmental microbiology. 2002;68(4):1561-1568. https://doi.org/10.1128/AEM.68.4.1561-1568.2002.
35. Helander IM, Alakomi HL, Latva-Kala K, MattilaSandholm T, Pol I, Smid EJ, et al. Characterization of the Action of Selected Essential Oil Components on GramNegative Bacteria. Journal of Agricultural and Food Chemistry. 1998;46(9):3590-3595. Doi:10.1021/jf980154m.
36. Omonijo FA, Ni L, Gong J, Wang Q, Lahaye L, Yang C. Essential oils as alternatives to antibiotics in swine production. Animal Nutrition. 2018;4(2):126-136. doi: 10.1016/j.aninu.2017.09.001.
37. Gostner JM, Ganzera M, Becker K, Geisler S, Schroecksnadel S, Überall F, et al. Lavender oil suppresses indoleamine 2,3-dioxygenase activity in human PBMC. BMC complementary and alternative medicine. 2014;14:503. https://doi.org/10.1186/1472- 6882-14-503.
38. Ameur E, Sarra ME, Takoua K, Mariem K, Nabil A, Lynen F, et al. Chemical composition of five Tunisian Pinus Species’ essential oils and effect of their blends on Otitis infection, Industrial Crops and Products. 2022;180:114688, https://doi.org/10.1016/j.indcrop.2022.114688.
39. Rath CC, Devi S, Dash SK, Mishra RK. Antibacterial potential assessment of jasmine essential oil against e. Coli. Indian journal of pharmaceutical sciences. 2008;70(2):238-241. https://doi.org/10.4103/0250- 474X.41465.
40. Ozaki MM, dos Santos M, Ribeiro WO, Chinellato de Azambuja Ferreira N, Picone CSF, Domínguez R, et al. Radish powder and oregano essential oil as nitrite substitutes in fermented cooked sausages, Food Research International. 2021;140:109855. https://doi.org/10.1016/j.foodres.2020.109855.
41. Liao M, Xiao JJ, Zhou LJ., Yao X, Tang F, Hua RM, et al. Chemical composition, insecticidal and biochemical effects of Melaleuca alternifolia essential oil on the Helicoverpa armigera. Journal of Applied Entomology, 2017.
42. Mashayekhi H, Mazhari M, Esmaeilipour O. Eucalyptus leaves powder, antibiotic and probiotic addition to broiler diets: effect on growth performance, immune response, blood components and carcass traits. Animal. 2018;12(10):2049-2055. The Animal Consortium 2018doi:10.1017/S17517311170037.
43. Khalafalla FA, Ali FHM, Hassan Abdel-Rahim HA. Quality improvement and shelf-life extension of refrigerated Nile tilapia (Oreochromis niloticus) fillets using natural herbs. Beni-Suef University Journal of Basic and Applied Sciences. 2015;4(1):33-40. https://doi.org/10.1016/j.bjbas.2015.02.005.
44. Khan M, Srivastava SK, Syamasundar KV, Singh M, Naqvi AA. Chemical composition of leaf and flower essential oil of Lantana camara from India. Flavour and Fragrance Journal. First published: 07 December 2001.
45. Ozogul Y, Kuley E, Ucar Y, Ozogul F. Antimicrobial Impacts of Essential Oils on Food Borne-Pathogens. Recent Patents on Food, Nutrition & Agriculture. 2015;7(1). Doi: 10.2174/2212798407666150615112153.









.jpg&w=3840&q=75)