Characteristics of Nutrition and Metabolism in Dogs and Cats
Domestic dogs and cats have evolved differentially in some aspects of nutrition, metabolism, chemical sensing, and feeding behavior. The dogs have adapted to omnivorous diets containing taurine-abundant meat and starch-rich plant ingredients. By contrast, domestic cats must consume animal-sourced foods for survival, growth, and development. Both dogs and cats synthesize vitamin C and many amino acids (AAs, such as alanine, asparagine, aspartate, glutamate, glutamine, glycine, proline, and serine), but have a limited ability to form de novo arginine and vitamin D3. Compared with dogs, cats have greater endogenous nitrogen losses and higher dietary requirements for AAs (particularly arginine, taurine, and tyrosine), B-complex vitamins (niacin, thiamin, folate, and biotin), and choline; exhibit greater rates of gluconeogenesis; are less sensitive to AA imbalances and antagonism; are more capable of concentrating urine through renal reabsorption of water; and cannot tolerate high levels of dietary starch due to limited pancreatic α-amylase activity. In addition, dogs can form sufficient taurine from cysteine (for most breeds); arachidonic acid from linoleic acid; eicosapentaenoic acid and docosahexaenoic acid from α-linolenic acid; all-trans-retinol from β-carotene; and niacin from tryptophan. These synthetic pathways, however, are either absent or limited in all cats due to (a) no or low activities of key enzymes (including pyrroline-5-carboxylate synthase, cysteine dioxygenase, ∆6-desaturase, β-carotene dioxygenase, and quinolinate phosphoribosyltransferase) and (b) diversion of intermediates to other metabolic pathways. Dogs can thrive on one large meal daily, select high-fat over low-fat diets, and consume sweet substances. By contrast, cats eat more frequently during light and dark periods, select high-protein over low-protein diets, refuse dry food, enjoy a consistent diet, and cannot taste sweetness. This knowledge guides the feeding and care of dogs and cats, as well as the manufacturing of their foods. As abundant sources of essential nutrients, animal-derived foodstuffs play important roles in optimizing the growth, development, and health of the companion animals.
Keywords Dogs · Cats · Nutrition · Metabolism · Feeding · Health · Animal-sourced foodstuffs
4.1 Introduction
4.3 Lipid Metabolism in Dogs and Cats
4.3.1 Overview of Lipid Metabolism
4.3.2 Lipid Metabolism in Dogs
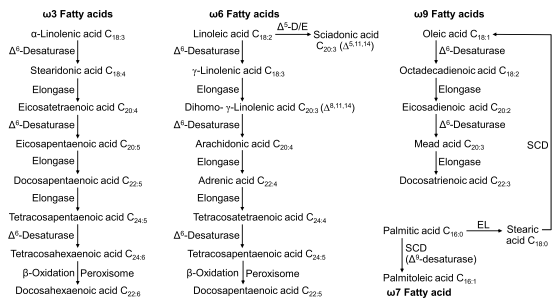
4.3.3 Lipid Metabolism in Cats
4.4 Carbohydrate Metabolism in Dogs and Cats
4.4.1 Overview of Carbohydrate Metabolism
4.4.2 Carbohydrate Metabolism in Dogs
4.4.3 Carbohydrate Metabolism in Cats
4.5 Protein and AA Metabolism in Dogs and Cats
4.5.1 Overview of Protein and AA Metabolism
4.5.2 Arginine Metabolism in Dogs and Cats
4.5.3 Cysteine Metabolism in Dogs and Cats
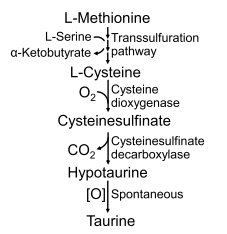
4.5.4 AA Imbalances and Antagonisms, as Well as Metabolic Adaptation to AA Intakes
4.5.5 AA Requirements of Dogs and Cats
4.6 Vitamin Metabolism in Dogs and Cats
4.6.1 Vitamin Metabolism in Dogs
4.6.2 Vitamin Metabolism in Cats
4.6.3 Anti-vitamin Factors in Foods
4.7 Mineral Metabolism in Dogs and Cats
4.7.1 Mineral Metabolism in Dogs
4.7.2 Mineral Metabolism in Cats
4.8 Water Metabolism in Dogs and Cats
4.8.1 Overview of Water Metabolism
4.8.2 Water Metabolism in Dogs
4.8.3 Water Metabolism in Cats
4.9 Feeding Behavior and Management of Dogs and Cats
4.9.1 General Considerations
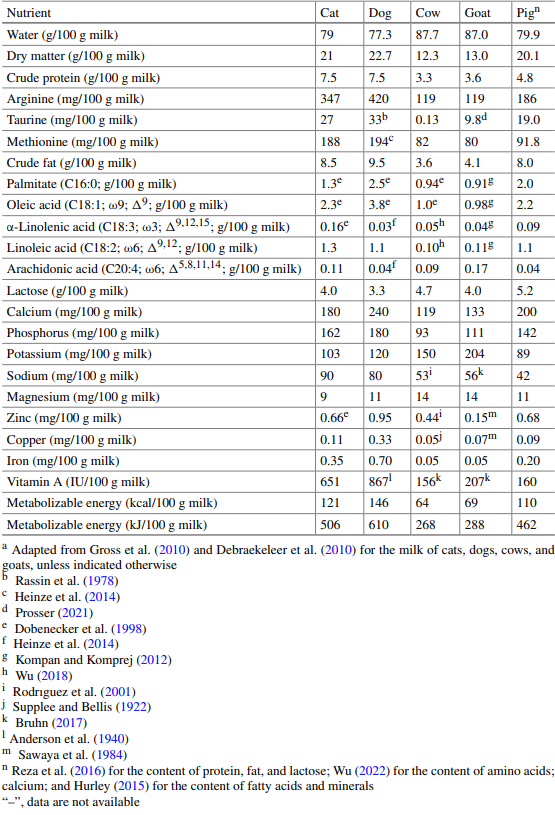
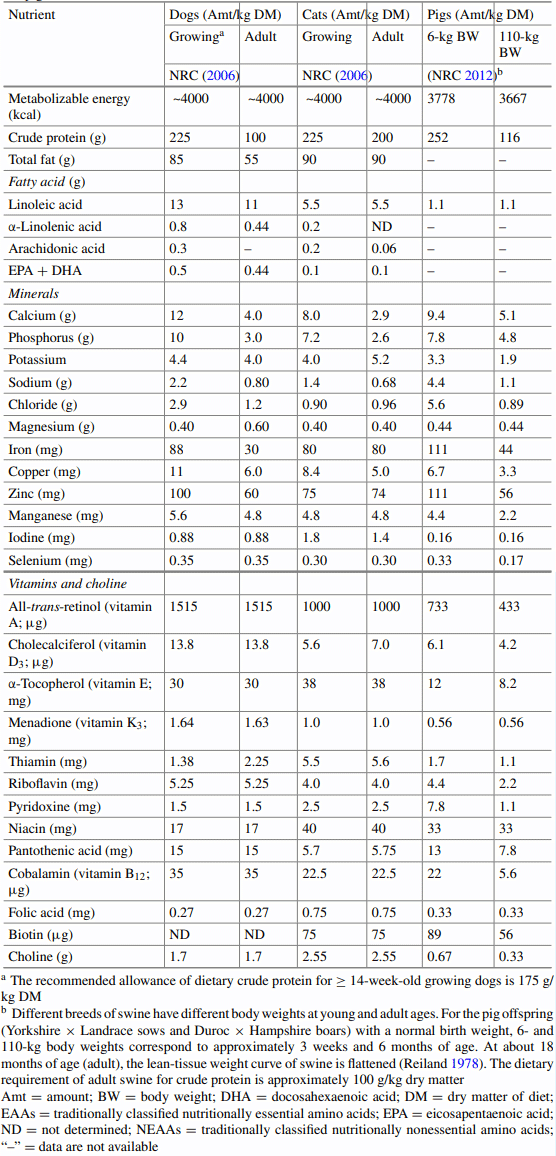
4.9.2 Weaning of Dogs and Cats
4.9.3 Food Selection of Dogs and Cats
4.9.4 Meal Frequency of Dogs and Cats
4.10 Conclusion and Perspectives
Anderson HD, Johnson BC, Arnold A (1940) The composition of dog’s milk. Am J Physiol 129:631– 634
Anderson RS (1982) Water balance in the dog and cat. J Small Anim Pract 23:588–598
Anderson PJ, Rogers QR, Morris JG (2002) Cats require more dietary phenylalanine or tyrosine for melanin deposition in hair than for maximal growth. J Nutr 132:2037–2042
Axelsson E, Ratnakumar A, Arendt M-L et al (2013) The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature 495:360–364
Backus RC, Cohen G, Pion PD, Good KL, Rogers QR, Fascetti AJ (2003) Taurine deficiency in Newfoundlands fed commercially available complete and balanced diets. J Am Vet Med Assoc 223:1130–1136
Backus RC, Ko KS, Fascetti AJ, Kittleson MD, MacDonald KA, Maggs DJ, Berg JR, Rogers QR (2006) Low plasma taurine concentration in Newfoundland dogs is associated with low plasma methionine and cyst(e)ine concentrations and low taurine synthesis. J Nutr 136:2525–2533
Badawy AA (2017) Kynurenine pathway of tryptophan metabolism: Regulatory and functional aspects. Int J Tryptophan Res 10:1178646917691938
Baker DH, Czarnecki-Maulden GL (1991) Comparative nutrition of cats and dogs. Annu Rev Nutr 11:239–263
Bartges JM, Cook M (1999) Influence of feeding conjugated linoleic acid on body composition in healthy adult cats. In: Proceedings 17th ACVIM. pp 729
Bataller N (1995) Risk factors and the debate of diet in canine gastric dilatation-volvulus. Vet Clin Nutr 2:87
Batchelor DJ, Al-Rammahi M, Moran AW, Brand JG, Li X, Haskins M, German AJ, ShiraziBeechey SP (2011) Sodium/glucose cotransporter-1, sweet receptor, and disaccharidase expression in the intestine of the domestic dog and cat: two species of different dietary habit. Am J Physiol 300:R67-75
Bauer JE (1997) Fatty acid metabolism in domestic cats (Felis catus) and cheetahs (Acinonyx jubatas). Proc Nutr Soc 56:1013–1024
Bauer JE (2007) Responses of dogs to dietary omega-3 fatty acids. J Am Vet Med Assoc 231:1657– 1661
Beauchamp GK, Maller O, Rogers JG Jr (1977) Flavor preferences in cats (Felis catus and Panthera sp). J Comp Physiol Psychol 91:1118–1127
Beliveau GP, Freedland RA (1982) Metabolism of serine, glycine and threonine in isolated cat hepatocytes Felis domestica. Comp Biochem Physiol B 71:13–18
Belo PS, Romsos DR, Leveille GA (1976) Influence of diet on glucose tolerance, on the rate of glucose utilization and on gluconeogenic enzyme activities in the dog. J Nutr 106:1465–1474
Bermingham EN, Thomas DG, Morris PJ, Hawthorne AJ (2010) Energy requirements of adult cats. Br J Nutr 103:1083–1093
Besch EL, Woods JE (1977) Heat production biorhythms of laboratory animals. Lab Anim Sci 27:54–59
Biourge V, Sergheraert R (2002) Hair pigmentation can be affected by diet in dogs. Proc Comp Nutr Soc 4:103–104
Bordoni A, Biagi PL, Turchetto E, Hrelia S (1988) Aging influence on delta-6-desaturase activity and fatty acid composition of rat liver microsomes. Biochem Int 17:1001–1009
Bradshaw JWS (2006) The evolutionary basis for the feeding behavior of domestic dogs (Canis familiaris) and cats (Felis catus). J Nutr 136:1927S-1931S
Bradshaw JWS, Goodwin D, Legrand-Defretin V, Nott HMR (1996) Food selection by the domestic cat, an obligate carnivore. Comp Biochem Physiol A 114:205–209
Bray EE, Zheng Z, Tolbert MK, McCoy BM, Dog Aging Project Consortium, Kaeberlein M, Kerr KF (2021) Once-daily feeding is associated with better health in companion dogs: results from the Dog Aging Project. bioRxiv preprint. https://doi.org/10.1101/2021.11.08.467616
Brody S, Procter RC, Ural S Ashworth US (1934) Growth and development with special reference to domestic animals. XXXIV, Basal metabolism, endogenous nitrogen, creatinine and neutral sulphur excretions as functions of body weight. Agric Exp Stn Res Bull No 220. University of Missouri, Columbia
Brooks D, Churchill J, Fein K et al (2014) AAHA weight management guidelines for dogs and cats. J Am Anim Hospital Assoc 50:1–11
Brosey BP, Hill RC, Scott KC (2000) Gastrointestinal volatile fatty acid concentrations and pH in cats. Am J Vet Res 61:359–361
Bruhn JC (2017) Dairy goat milk composition. https://drinc.ucdavis.edu/goat-dairy-foods/dairygoat-milk-composition. Accessed 5 Oct 2021
Bryan DL, Hart P, Forsyth K, Gibson R (2001) Incorporation of alpha-linolenic acid and linoleic acid into human respiratory epithelial cell lines. Lipids 36:713–717
Buff PR, Carter RA, Bauer JE, Kersey JH (2014) Natural pet food: a review of natural diets and their impact on canine and feline physiology. J Anim Sci 92:3781–3791
Burns RA, Milner JA, Corbin JE (1981) Arginine: An indispensable amino acid for mature dogs. J Nutr 111:1020–1024
Camara A, Verbrugghe A, Cargo-Froom C, Hogan K, DeVries TJ, Sanchez A, Robinson LE, Shoveller AK (2020) The daytime feeding frequency affects appetite-regulating hormones, amino acids, physical activity, and respiratory quotient, but not energy expenditure, in adult cats fed regimens for 21 days. PLoS ONE 15:e0238522
Carciofi AC, Takakura FS, de-Oliveira LD, Teshima E, Jeremias JT, Brunetto MA, Prada F (2008) Effects of six carbohydrate sources on dog diet digestibility and post-prandial glucose and insulin response. J Anim Physiol Anim Nutr 92:326–336
Case LP, Daristotle L, Hayek MG, Raasch MF (2011) Canine and feline nutrition, 3rd edn. Mosby, Maryland Heights, Missouri
Cavanaugh SM, Cavanaugh RP, Gilbert GE, Leavitt EL, Ketz is JK, Vieira AB (2021) Shortterm amino acid, clinicopathologic, and echocardiographic findings in healthy dogs fed a commercial plant-based diet. PLoS ONE 16:e0258044
Che DS, Nyingwa PS, Ralinala KM, Maswanganye GMT, Wu G (2021) Amino acids in the nutrition, metabolism, and health of domestic cats. Adv Exp Med Biol 1285:217–231
Chew RM (1965) Water metabolism of mammals. In: Mayer WV, van Gelder RG (eds) Physiological mammalogy: mammalian reactions to stressful environments. Academic Press, New York, pp 43–178
Chiocchetti R, Galiazzo G, Giancola F, Tagliavia C, Bernardini C, Forni M, Pietra M (2021) Localization of the serotonin transporter in the dog intestine and comparison to the rat and human intestines. Front Vet Sci 8:802479
Churchill JA, Eirmann L (2021) Senior pet nutrition and management. Vet Clin North Am Sm Anim Pract 51:635–651
Clark MH, Ferguson DC, Bunick D, Hoenig M (2013) Molecular and histological evidence of brown adipose tissue in adult cats. Vet J 195:66–72
Cook NE, Kane E, Rogers QR, Morris JG (1985) Self selection of dietary casein and soy-protein by the cat. Physiol Behav 34:583–594
Cook NE, Rogers QR, Morris JG (1996) Acid-base balance affects dietary choice in cats. Appetite 26:175–192
Croniger CM, Olswang Y, Reshef L, Kalhan SC, Tilghman SM, Hanson RW (2002) Phosphoenolpyruvate carboxykinase revisited: insight into its metabolic role. Biochem Mol Bil Educ 30:14–20
Czarnecki GL, Baker DH (1984) Urea cycle function in the dog with emphasis on the role of arginine. J Nutr 114:581–590
Davis TA, Nguyen HV, Garcia-Bravo R, Fiorotto ML, Jackson EM, Lewis DS, Lee DR, Reeds PJ (1994a) Amino acid composition of human milk is not unique. J Nutr 124:1126–1132
Davis TA, Nguyen HV, Garcia-Bravo R, Fiorotto ML, Jackson EM, Reeds PJ (1994b) Amino acid composition of the milk of some mammalian species changes with stage of lactation. Br J Nutr 72:845–853
De Bruijne JJ, van den Brom WE (1986) The effect of long-term fasting on ketone body metabolism in the dog. Comp Biochem Physiol B 83:391–395
Debraekeleer J, Gross KL, Zicker SC (2010) Feeding nursing and orphaned puppies from birth to weaning. In: Thatcher CD, Remillard RL, Roudebush P (eds) Small Animal clinical nutrition Hand MS. Mark Morris Institute, Topeka, KS, pp 295–309
DeNapoli JS, Dodman NH, Shuster L, Rand WM, Gross KL (2000) Effect of dietary protein content and tryptophan supplementation on dominance aggression, territorial aggression, and hyperactivity in dogs. J Am Vet Med Assoc 217:504–508
Dillon EL, Wu G (2021) Cortisol enhances ctrulline synthesis from proline in enterocytes of suckling piglets. Amino Acids 53:1957–1966
Dobenecker B, Zottmann B, Kienzle E, Zentek J (1998) Investigations on milk composition and milk yield in queens. J Nutr 128:2618S-2619S
Dodman NH, Reisner I, Shuster L, Rand W, Luescher UA, Robinson I, Houpt KA (1996) Effect of dietary protein content on behavior in dogs. J Am Vet Med Assoc 208:376–379
Dunbar BL, Bauer JE (2002) Conversion of essential fatty acids by delta-6 desaturase in dog liver microsomes. J Nutr 132:1701S-1703S
Eyjolfson V, Spriet LL, Dyck DJ (2004) Conjugated linoleic acid improves insulin sensitivity in young, sedentary humans. Med Sci Sports Exerc 36:814–820
Eyre R, Trehiou M, Marshall E, Carvell-Miller L, Goyon A, McGrane S (2022) Aging cats prefer warm food. J Vet Behav 47:86–92
Félix AP, Gabeloni LR, Brito CBM, Oliveira SG, Silva AVF, Maiorka A (2012) Effect of βmannanase on the digestibility of diets with different protein sources in dogs determined by different methodologies. J Anim Sci 90:3060–3067
Feng BC, Li J, Kliegman RM (1996) Transcription of hepatic cytosolic phosphoenolpyruvate carboxykinase gene in newborn dogs. Biochem Mol Med 59:13–19
Finco DR, Adams DD, Crowelle WA, Stattelman AJ, Brown SA, Barsanti JA (1986) Food and water intake and urine composition in cats: influence of continuous versus periodic feeding. Am J Vet Res 47:1638–1642
Flaim D (2019) Can dogs drink too much water? The dangers of water intoxication. American Kennel Club. https://www.akc.org/expert-advice/health/can-dogs-drink-much-water-dangers-water-int oxication. Accessed 12 Jan 2022
Galim EB, Hruska K, Bier DM, Matthews DE, Haymond MW (1980) Branched-chain amino acid nitrogen transfer to alanine in vivo in dogs. J Clin Invest 66:1295–1304
Gershoff SN, Andrus SB, Hegsted DM, Lentini EA (1957) Vitamin A deficiency in cats. Lab Invest 6:227–240
Gil F, Arencibia A, García V, Ramírez G, Vázquez JM (2018) Anatomic and magnetic resonance imaging features of the salivary glands in the dog. Anat Histol Embryol 47:551–559
Glynn EL, Fry CS, Drummond MJ, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB (2010) Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J Nutr 140:1970–1976
Green AS, Ramsey JJ, Villaverde C, Asami DK, Wei A, Fascetti AJ (2008) Cats are able to adapt protein oxidation to protein intake provided their requirement for dietary protein is met. J Nutr 138:1053–1060
Greig RA, Gnaedinger RH (1971) Occurrence of thiaminase in some common aquatic animals of the United States and Canada. Special Scientific Report—Fish. US Dept Commer Natl Mar Fish Serv 631:1–7
Gross KL, Becvarova I, Debraekeleer J (2010) Feeding nursing and orphaned kittens from birth to weaning. In: Thatcher CD, Remillard RL, Roudebush P (eds) Small Animal clinical nutrition Hand MS. Mark Morris Institute, Topeka, KS, pp 415–427
Hall JA, Vondran JC, Vanchina MA, Jewell DE (2018a) When fed foods with similar palatability, healthy adult dogs and cats choose different macronutrient compositions. J Exp Biol 221:jeb173450
Hall JA, Jackson MI, Vondran JC, Vanchina MA, Jewell DE (2018b) Comparison of circulating metabolite concentrations in dogs and cats when allowed to freely choose macronutrient intake. Biol Open 7:bio036228
Hammer VA, Rogers QR, Freedland RA (1996) Threonine is catabolized by L-threonine 3- dehydrogenase and threonine dehydratase in hepatocytes from domestic cats (Felis domestica). J Nutr 126:2218–2226
Hanson RW, Garber AJ (1972) Phosphoenolpyruvate carboxykinase. I. Its role in gluconeogenesis. Am J Clin Nutr 25:1010–1021
Hargrove DM, Morris JG, Rogers QR (1994) Kittens choose a high leucine diet even when isoleucine and valine are the limiting amino acids. J Nutr 124:689–693
Harper AE, Miller RM, Block KP (1984) Branched-chain amino acid metabolism. Annu Rev Nutr 4:409–454
Hart BL, Hart LA, Thigpen AP, Willits NH (2020) Assisting decision-making on age of neutering for 35 breeds of dogs: associated joint disorders, cancers, and urinary incontinence. Front Vet Sci 7:388
Hawking F, Lobban MC, Gammage K, Worms MJ (1971) Circadian rhythms (activity, temperature, urine and microfilariae) in dog, cat, hen, duck, thamnomys and gerbillus. Int J Cycle Res 2:455– 473
Hazewinkel HAW, van den Brom WE, van’t Klooster A, Voorhout G, van Wees A (1991) Calcium metabolism in Great Dane dogs fed diets with various calcium and phosphorus contents. J Nutr 121:S99–S106
He W, Connolly ED, Wu G (2024) Characteristics of the digestive tract of dogs and cats. Adv Exp Med Biol 1446:15–38. https://doi.org/10.1007/978-3-031-54192-6_2
He WL, Wu G (2022) Oxidation of amino acids, glucose, and fatty acids as metabolic fuels in enterocytes of developing pigs. Amino Acids 54:1025–1039
He WL, Posey EA, Steele CC, Savell JW, Bazer FW, Wu G (2023) Dietary glycine supplementation enhances postweaning growth and meat quality of pigs with intrauterine growth restriction. J Anim Sci 101:skadskad354
Hedhammar A, Wu FM, Krook L, Schryver HF, de LaHunta A, Wahlen JP, Kallfelz FA, Nunez EA, Hintz HF, Sheffy BE, Ryan GD (1974) Overnutrition and skeletal disease. An experimental study in growing Great Dane dogs. Cornell Vet 64 (suppl 5):1–160
Heinze CR, Larsen JA, Kass PH, Fascetti AJ (2009) Plasma amino acid and whole blood taurine concentrations in cats eating commercially prepared diets. Am J Vet Res 70:1374–1382
Heinze CR, Freeman LM, Martin CR, Power ML, Fascetti AJ (2014) Comparison of the nutrient composition of commercial dog milk replacers with that of dog milk. J Am Vet Med Assoc 244:1413–1422
Hendriks WH, Moughan PJ, Tarttelin MF (1996) Gut endogenous nitrogen and amino acid excretions in adult domestic cats fed a protein-free or an enzymatically hydrolyzed casein-based diet. J Nutr 126:955–962
Hendricks WH, Moughan PJ, Tarttelin MF (1997) Urinary excretion of endogenous nitrogen metabolites in adult domestic cats using a protein-free diet and the regression technique. J Nutr 127:623–629
Hendriks WH, Sritharan K, Hodgkinson SM (2002) Comparison of the endogenous ileal and faecal amino acid excretion in the dog (Canis familiaris) and the rat (Rattus rattus) determined under protein-free feeding and peptide alimentation. J Anim Physiol Anim Nutr 86:333–341
Hendriks WH, Vather R, Rutherfurd SM, Weidgraaf K, Rutherfurd-Markwick KJ (2004) Urinary isovalthine excretion in adult cats is not gender dependent or increased by oral leucine supplementation. J Nutr 134:2114S-2116S
Hendriks WH, Rutherfurd-Markwick KJ, Weidgraaf K, Morton RH, Rogers QR (2008) Urinary felinine excretion in intact male cats is increased by dietary cystine. Br J Nutr 100:801–809
Hewson-Hughes AK, Gilham MS, Upton S, Colyer A, Butterwick R, Miller AT (2011) The effect of dietary starch level on postprandial glucose and insulin concentrations in cats and dogs. Br J Nutr 106:S105–S109
Hewson-Hughes AK, Colyer A, Simpson SJ, Raubenheimer D (2016) Balancing macronutrient intake in a mammalian carnivore: disentangling the influences of flavour and nutrition. R Soc Open Sci 3:160081
Hill RC (1998) The nutritional requirements of exercising dogs. J Nutr 128:2686S-2690S
Hill RC, Scott KC (2004) Energy requirements and body surface area of cats and dogs. JAVMA 225:689–694
Holloway B, Stribling D, Freeman S, Jamieson L (1985) The thermogenic role of adipose tissue in the dog. Int J Obesity 9:423–432
Holowaty MNH, Lees MJ, Abou Sawan S, Paulussen KJM, Jäger R, Purpura M, Paluska SA, Burd NA, Hodson N, Moore DR (2023) Leucine ingestion promotes mTOR translocation to the periphery and enhances total and peripheral RPS6 phosphorylation in human skeletal muscle. Amino Acids 55:253–261
Hou YQ, Wu G (2017) Nutritionally nonessential amino acids: a misnomer in nutritional sciences. Adv Nutr 8:137–139
Hou YQ, Yin YL, Wu G (2015) Dietary essentiality of “nutritionally nonessential amino acids” for animals and humans. Exp Biol Med 240:997–1007
Hou YQ, Yao K, Yin YL, Wu G (2016) Endogenous synthesis of amino acids limits growth, lactation and reproduction of animals. Adv Nutr 7:331–342
Hou YQ, Wu ZL, Dai ZL, Wang GH, Wu G (2017) Protein hydrolysates in animal nutrition: Industrial production, bioactive peptides, and functional significance. J Anim Sci Biotechnol 8:24
Hou YQ, He WL, Hu SD, Wu G (2019) Composition of polyamines and amino acids in plant-source foods for human consumption. Amino Acids 51:1153–1165
Houston DM, Moore AEP, Favrin MG, Brent Hoff B (2003) Feline urethral plugs and bladder uroliths: a review of 5484 submissions 1998–2003. Can Vet J 44:974–977
How KL, Hazewinkel HAW, Mol JA (1994) Dietary vitamin D dependence of cat and dog due to inadequate cutaneous synthesis of vitamin D. Gen Comp Endocrinol 96:12–18
Hu SD, He WL, Wu G (2021) Hydroxyproline in animal metabolism, nutrition, and cell signaling. Amino Acids 54:513–528
Humbert B, Martin L, Dumon H, Darmaun D, Nguyen P (2002) Dietary protein level affects protein metabolism during the postabsorptive state in dogs. J Nutr 132:1676S – 1678
Hurley WL (2015) Composition of sow colostrum and milk. In: Farmer C (ed) The gestating and lactating sow. Wageningen Academic Publishers, Wageningen, The, Netherlands, pp 193–229
Iyer MS, Paszkiewicz RL, Bergman RN, Richey JM, Woolcott OO, Asare-Bediako I, Wu Q, Kim SP, Stefanovski D, Kolka CM, Clegg DJ, Kabir M (2019) Activation of NPRs and UCP1- independent pathway following CB1R antagonist treatment is associated with adipose tissue beiging in fat-fed male dogs. Am J Physiol 317:E535-547
Jacobsen JG, Smith LH (1968) Biochemistry and physiology of taurine and taurine derivatives. Physiol Rev 48:424–511
Jobgen WJ, Meininger CJ, Jobgen SC, Li P, Lee M-J, Smith SB, Spencer TE, Fried SK, Wu G (2009) Dietary L-arginine supplementation reduces white-fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats. J Nutr 139:230–237
Jobgen WS, Wu G (2022a) L-Arginine increases AMPK phosphorylation and the oxidation of energy substrates in hepatocytes, skeletal muscle cells, and adipocytes. Amino Acids 54:1553–1568
Jobgen WS, Wu G (2022b) Dietary L-arginine supplementation increases the hepatic expression of AMP-activated protein kinase in rats. Amino Acids 54:1569–1584
Jobgen WS, Lee M-J, Fried SK, Wu G (2023) L-Arginine supplementation regulates energysubstrate metabolism in skeletal muscle and adipose tissue of diet-induced obese rats. Exp Biol Med 248:209–216
Kane E, Rogers QR, Morris JG, Leung PMB (1981) Feeding behavior of the cat fed laboratory and commercial diets. Nutr Res 1:499–507
Kaplan JL, Stern JA, Fascetti AJ, Larsen JA, Skolnik H, Peddle GD, Kienle RD, Waxman A, Cocchiaro M, Gunther-Harrington CT, Klose T, LaFauci K, Lefbom B, Lamy MM, Malakoff R, Nishimura S, Oldach M, Rosenthal S, Stauthammer C, O’Sullivan L, Visser LC, Williams R, Ontiveros E (2018) Taurine deficiency and dilated cardiomyopathy in golden retrievers fed commercial diets. PLoS ONE 13:e0209112
Kealy RD (1999) Factors influencing lean body mass in aging dogs. Comp Cont Edu Small Anim Pract 2:34–37
Kealy RD, Lawler DF, Ballam JM et al (2002) Effects of diet restriction on life span and 358 age-related changes in dogs. J Am Vet Med Assoc 220:1315–1320
Kendall PT, Blaze SE, Holme DW (1982) Assessment of endogenous nitrogen output in adult dogs of contrasting size using a protein-free diet. J Nutr 112:1281–1286
Kettelhut IC, Foss MC, Migliorini RH (1980) Glucose homeostasis in a carnivorous animal (cat) and in rats fed a high-protein diet. Am J Physiol 239:R437-444
Khanna C, Boermans HJ, Wilcock B (1997) Fatal hypernatremia in a dog from salt ingestion. J Am Anim Hosp Assoc 33:113–116
Kienzle E (1993a) Carbohydrate metabolism of the cat. 1. Activity of amylase in the gastrointestinal tract of the cat. J Anim Physiol Anim Nutr 69:92–101
Kienzle E (1993b) Carbohydrate metabolism of the cat. 2. Digestion of starch. J Anim Physiol Anim Nutr 69:102–114
Kirk RW, Bistner SI (1981) Handbook of veterinary procedures and emergency treatment. W.B. Saunders, Philadelphia, p 582
Kittleson MD, Keene B, Pion PD, Loyer CG (1997) Results of the multicenter spaniel trial (MUST): taurine- and carnitine-responsive dilated cardiomyopathy in American cocker spaniels with decreased plasma taurine concentration. J Vet Intern Med 11:204–211
Kleiber M (1961) The fire of life. Wiley & Sons, New York Knopf K, Sturman JA, Armstrong M, Hayes KC (1978) Taurine: an essential nutrient for the cat. J Nutr 108:773–778
Kompan D, Komprej A (2012) The effect of fatty acids in goat milk on health. In: Chaiyabutr N (ed) Milk production, IntechOpen, London, UK Krehl WA (1981) Discovery of the effect of tryptophan on niacin deficiency. Fed Proc 40:1527–1530
Kumazawa T, Kurihara K (1990a) Large enhancement of canine taste responses to sugars by salts. J Gen Physiol 95:1007–1018
Kumazawa T, Kurihara K (1990b) Large synergism between monosodium glutamate and 59– nucleotides in canine taste nerve responses. Am J Physiol 259:R420–R426
Kuo ZY (1967) The dynamics of behaviour development: an epigenetic view. Random House, New York
Ladd M, Raisz LG (1949) Response of the normal dog to dietary sodium chloride. Am J Physiol 159:149–152
Laflamme D (2008a) Effect of diet on loss and preservation of lean body mass in aging dogs and cats. Companion Animal Nutrition Summit, May 3–5, 2008, Charleston, South Carolina. pp 41–46
Laflamme DP (2008b) Pet food safety: dietary protein. Top Companion Anim Med 23:154–157
Laflamme DP, Hannah SS (2010) Pet dogs and vats. In: Pond KR (ed) Introduction to animal science (pond WG. John Wiley & Sons, New York, pp 526–552
Laflamme DP, Hannah SS (2013) Discrepancy between use of lean body mass or nitrogen balance to determine protein requirements for adult cats. J Feline Med Surg 15:691–697
Laflamme D, Danièlle G-M (2014) Nutrition of aging cats. Vet Clin North Am Sm Anim Pract 44:761–774
Lambie JG, Pezzali JG, Richards TL, Ellis JL, Verbrugghe A, Shoveller AK (2024) Phenylalanine requirements using the direct amino acid oxidation technique, and the effects of dietary phenylalanine on food intake, gastric emptying, and macronutrient metabolism in adult cats. J Anim Sci 102:skae009
Landsberg G, Milgram B, Mougeot I, Kelly S, de Rivera C (2017) Therapeutic effects of an alphacasozepine and L-tryptophan supplemented diet on fear and anxiety in the cat. J Feline Med Surg 19:594–602
Larsen TM, Toubro A, Astrup A (2003) Efficacy and safety of dietary supplements containing CLA for the treatment of obesity: evidence from animal and human studies. J Lipid Res 44:2234–2241
Larsen JA, Oberbauer AM (2023) Dog nutrition. In: Phillips CJC (ed) Encyclopedia of Animal Nutrition, 2nd edition. CABI, Wallingford, pp 176–179
Latimer HB (1938) The weights of the brain and of its parts, of the spinal cord and of the eyeballs in the adult cat J Comp Neurol 68:395–404
Latimer HB (1967) Variability in body and organ weights in the newborn dog and cat compared with that in the adult. Anat Red 157:449–456
Lawler DF, Larson BT, Ballam JM, Smith GK, Biery DN, Evans RH, Greeley EH, Segre M, Stowe HD, Kealy RD (2008) Diet restriction and ageing in the dog: major observations over two decades. Br J Nutr 99:793–805
Legrand-Defretin V (1994) Differences between cats and dogs: a nutritional view. Proc Nutr Soc 53:15–24
Lemaitre RN, King IB, Sotoodehnia N, Rea TD, Raghunathan TE, Rice KM, Lumley TS, Knopp RH, Cobb LA, Copass MK, Siscovick DS (2009) Red blood cell membrane alpha-linolenic acid and the risk of sudden cardiac arrest. Metabolism 58:534–540
Leung YB, Cave NJ, Heiser A, Edwards PJB, Godfrey AJR, Wester T (2020) Metabolic and immunological effects of intermittent fasting on a ketogenic diet containing medium-chain triglycerides in healthy dogs. Front Vet Sci 6:480
Levillain O, Parvy P, Hus-Citharel A (1996) Arginine metabolism in cat kidney. J Physiol 491:471– 477
Li P, Wu G (2018) Roles of dietary glycine, proline and hydroxyproline in collagen synthesis and animal growth. Amino Acids 50:29–38
Li P, Wu G (2020) Composition of amino acids and related nitrogenous nutrients in feedstuffs for animal diets. Amino Acids 52:523–542
Li P, Wu G (2022) Functional molecules of intestinal mucosal products in animal nutrition and health. Adv Exp Med Biol 1354:263–277
Li P, Wu G (2023a) Amino acid nutrition and metabolism in domestic cats and dogs. J Anim Sci Biotechnol 14:19
Li P, Wu G (2023b) Composition of nutrients in rendered animal-sourced feedstuffs. In: Phillips C (ed) Encyclopedia of animal nutrition, 2nd edn. CABI, Wallingford, Oxon, UK, pp 576–579
Li X, Li W, Wang H, Cao J, Maehashi K, Huang L, Bachmanov AA, Reed DR, LegrandDefretin V, Beauchamp GK (2005) Pseudogenization of a sweet-receptor gene accounts for cats’ indifference toward sugar. PLoS Genet 1:e27-35
Li X, Li W, Wang H, Bayley DL, Cao J, Reed DR, Bachmanov AA, Huang L, Legrand-Defretin V, Beauchamp GK, Brand JG (2006) Cats lack a sweet taste receptor. J Nutr 136(Suppl 7):1932S1934S
Li XL, Zheng SX, Jia SC, Song F, Zhou CP, Wu G (2020) Oxidation of energy substrates in tissues of largemouth bass (Micropterus salmoides). Amino Acids 52:1017–1032
Li P, He WL, Wu G (2021a) Composition of amino acids in foodstuffs for humans and animals. Adv Exp Med Biol 1332:189–210
Li XY, Zheng SX, Wu G (2021b) Nutrition and functions of amino acids in fish. Adv Exp Med Biol 1285:133–168
Li XY, Han T, Zheng SX, Wu G (2021c) Nutrition and functions of amino acids in aquatic crustaceans. Adv Exp Med Biol 1285:169–197
Li P, He WL, Wu G (2021d) Composition of amino acids in foodstuffs for humans and animals. Adv Exp Med Biol 1332:189–209
Li XY, He WL, Wu G (2023) Dietary glycine supplementation enhances the growth performance of hybrid striped bass (Morone saxatilis ~× Morone chrysops |) fed soybean meal-based diets. J Anim Sci 101:skad345
Lichtenberger M (2021) Thiaminase and its role in predatory pet fish (and other piscivores) nutrition. http://www.wetwebmedia.com/ca/volume_6/volume_6_1/thiaminase.htm: Accessed 19 Sept 2021
Loftus JP, Yazwinski M, Milizio JG, Wakshlag JJ (2014) Energy requirements for racing endurance sled dogs. J Nutr Sci 3:e34
Loncar D, Afzelius BA (1989) Ontogenetical changes in adipose tissue of the cat: convertible adipose tissue. J Ultrastruct Mol Struct Res 102:9–23
Macdonald DW, Apps PJ (1978) The social behaviour of a group of semi-dependent farm cats, Felis catus: a progress report. Carnivore Genet Newslett 3:256–268
MacDonald ML, Rogers QR, Morris JG (1985) Aversion of the cat to dietary medium-chain triglycerides and caprylic acid. Physiol Behav 35:371–375
Mansilla WD, Gorman A, Fortener L, Shoveller AK (2018) Dietary phenylalanine requirements are similar in small, medium, and large breed adult dogs using the direct amino acid oxidation technique. J Anim Sci 96:3112–3120
Mansilla WD, Templeman JR, Fortener L, Shoveller AK (2020a) Minimum dietary methionine requirements in Miniature Dachshund, Beagle, and Labrador Retriever adult dogs using the indicator amino acid oxidation technique. J Anim Sci 98:skaa324
Mansilla WD, Fortener L, Templeman JR, Shoveller AK (2020b) Adult dogs of different breed sizes have similar threonine requirements as determined by the indicator amino acid oxidation technique. J Anim Sci 98:skaa066
McCauley SR, Clark SD, Quest BW, Streeter RM, Oxford EM (2020) Review of canine dilated cardiomyopathy in the wake of diet-associated concerns. J Anim Sci 98:skaa155
McCue MD (2010) Starvation physiology: reviewing the different strategies animals use to survive a common challenge. Comp Biochem Physiol A 156:1–18
McDowell LR (1989) Vitamin C: vitamins in animal nutrition. San Diego, Academic Press, pp 365–387
McGeachin RL, Akin JR (1979) Amylase levels in the tissues and body fluids of the domestic cat (Felis catus). Comp Biochem Physiol B 63:437–439
Middleton RP, Lacroix S, Scott-Boyer MP, Dordevic N, Kennedy AD, Slusky AR, Carayol J, Petzinger-Germain C, Beloshapka A, Kaput J (2017) Metabolic differences between dogs of different body sizes. J Nutr Metab 2017:4535710
Mitchell WK, Phillips BE, Williams JP, Rankin D, Lund JN, Wilkinson DJ, Smith K, Atherton PJ (2015) The impact of delivery profile of essential amino acids upon skeletal muscle protein synthesis in older men. Clinical efficacy of pulse versus bolus supply. Am J Physiol 309:E450- 457
Miyazaki M, Yamashita T, Taira H, Suzuki A (2008) The biological function of cauxin, a major urinary protein of the domestic cat. In: Hurst JL, Beynon RJ, Roberts SC, Wyatt TD (eds) Chemical signals in vertebrates, vol 11. Springer, New York, pp 51–60
Montserrat-Malagarriga M, Castillejos L, Salas-Mani A, Torre C, Martín-Orúe SM (2024) The impact of fiber source on digestive function, fecal microbiota, and immune response in adult dogs. Animals 14:196
Morris JG (1999) Ineffective vitamin D synthesis in cats is reversed by an inhibitor of 7-dehydrocholestrol-∆7-reductase. J Nutr 129:903–908
Morris JG, Rogers QR, Pacioretty LM (1990) Taurine: an essential dietary nutrient for cats. J Small Anim Pract 31:502–509
Morris JG (2002) Idiosyncratic nutrient requirements of cats appear to be diet-induced evolutionary adaptations. Nutr Res Rev 15:153–168
Morris JG, Rogers QR (1978) Arginine: an essential amino acid for the cat. J Nutr 108:1944–1953
Morris JG, Trudell J, Pencovic T (1977) Carbohydrate digestion by the domestic cat (Felis catus). Br J Nutr 37:365–373
Mugford RA (1977) External influences on the feeding of carnivores. In: Maller O (ed) The chemical senses and nutrition (Kare MR. Academic Press, New York, pp 25–50
Mugford RA, Thorne C (1980) Comparative studies of meal patterns in pet and laboratory housed dogs and cats. In: Anderson RS (ed) Nutrition of the dog and cat. Pergamon Press, Oxford, UK, pp 3–14
National Research Council (NRC (2006) Nutrient requirements of dogs and cats. National Academies Press, Washington DC
National Research Council (NRC (2012) Nutrient requirements of swine. National Academies Press, Washington DC
Nogueira JPDS, He F, Mangian HF, Oba PM, de Godoy MRC (2019) Dietary supplementation of a fiber-prebiotic and saccharin-eugenol blend in extruded diets fed to dogs. J Anim Sci 97:4519–4531
Oberbauer AM, Larsen JA (2021) Amino acids in dog nutrition and health. Adv Exp Med Biol 1285:199–216
Oliveira R, HaeseI D, Kill JL, Lima A, Malini PV, Thompson GR (2016) Palatability of cat food with sodium pyrophosphate and yeast extract. Ciência Rural 46:2202–2205
Pawlosky R, Barnes A, Salem N Jr (1994) Essential fatty acid metabolism in the feline: relationship between liver and brain production of long-chain polyunsaturated fatty acids. J Lipid Res 35:2032–2040
Pekel AY, Mülazımo ˘glu SB, Acar N (2020) Taste preferences and diet palatability in cats. J Appl Anim Res 48:281–292
Pion PD, Kittleson MD, Rogers QR, Morris JG (1987) Myocardial failure in cats associated with low plasma taurine: a reversible cardiomyopathy. Science 237:764–768
Pion PD, Kittleson MD, Thomas WP, Skiles ML, Rogers QR (1992) Clinical findings in cats with dilated cardiomyopathy and relationship of findings to taurine deficiency. J Am Vet Med Assoc 201:267–274
Prosser CG (2021) Compositional and functional characteristics of goat milk and relevance as a base for infant formula. J Food Sci 86:257–265
Rabin B, Nicolosi RJ, Hayes KC (1976) Dietary influence on bile acid conjugation in the cat. J Nutr 106:1241–1246
Randall W, Johnson RF, Randall S, Cunningham JT (1985) Circadian rhythms in food intake and activity in domestic cats. Behav Neurosci 99:1162–1175
Ranz D, Gutbrod F, Corinna Eule C, Kienzle E (2002) Nutritional lens opacities in two litters of newfoundland dogs. J Nutr 132:1688S-1689S
Rassin DK, Sturman JA, Gaull GE (1978) Taurine and other free amino acids in milk of man and other mammals. Early Hum Dev 2:l–13
Reiland S (1978) Growth and skeletal development of the pig. Acta Radiol 358(Suppl):15–22
Rezaei R, Wu G (2022) Branched-chain amino acids regulate intracellular protein turnover in porcine mammary epithelial cells. Amino Acids 54:1491–1504
Rezaei R, Wu ZL, Hou YQ, Bazer FW, Wu G (2016) Amino acids and mammary gland development: nutritional implications for neonatal growth. J Anim Sci Biotechnol 7:20
Riond JL, Stiefel M, Wenk C, Wanner M (2003) Nutrition studies on protein and energy in domestic cats. J Anim Physiol Anim Nutr (berl) 87:221–228
Rivera NLM, Félix AP, Ferreira FM, da Silva AVF, Maiorka A (2011) Body measurements and serum lipid profile of overweight adult dogs fed diet with containing conjugated linoleic acid. Ciência Rural 41:2020–2025
Rivers JPW, Sinclair AJ, Crawford MA (1975) Inability of the cat to desaturate essential fatty acids. Nature 258:171–173
Robertson WG, Jones JS, Heaton MA, Stevenson AE, Markwell PJ (2002) Predicting the crystallization potential of urine from cats and dogs with respect to calcium oxalate and magnesium ammonium phosphate (struvite). J Nutr 132:1637S-1641S
Rodrıguez EMR, Alaejos MS, Romero CD (2001) Mineral concentrations in cow’s milk from the canary Island. J Food Compost Anal 14:419–430
Rogers QR, Morris JG (1979) Essentiality of amino acids for the growing kitten. J Nutr 109:718–723
Rogers QR, Phang JM (1985) Deficiency of pyrroline-5-carboxylate synthase in the intestinal mucosa of the cat. J Nutr 115:146–150
Rogers QR, Taylor TP, Morris JG (1998) Optimizing dietary amino acid patterns at various levels of crude protein for cats. J Nutr 128:2577S-2580S
Rogers QR, Morris JG, Freedland RA (1977) Lack of hepatic enzymatic adaptation to low and high levels of dietary protein in the adult cat. Enzyme 22:348–356
Rogers QR, Wigle AR, Laufer A, Castellanos VH, Morris JG (2004) Cats select for adequate methionine but not threonine. J Nutr 134:2046S-2049S
Romsos DR, Ferguson D (1983) Regulation of protein intake in adult dogs. J Am Vet Med Assoc 182:41–43
Romsos DR, Palmer HJ, Muiruri KL, Bennink MR (1981) Influence of a low carbohydrate diet on performance of pregnant and lactating dogs. J Nutr 111:678–689
Russell K, Murgatroyd PR, Batt RM (2002) Net protein oxidation is adapted to dietary protein intake in domestic cats (Felis silvestris catus). J Nutr 132:456–460
Rutherfurd-Markwick KJ, Rogers QR, Hendriks WH (2005) Mammalian isovalthine metabolism. J Anim Physiol Anim Nutr 89:1–10
Sawaya WN, Khalil JK, A F Al-Shalhat AF (1984) Mineral and vitamin content of goat’s milk. J Am Diet Assoc 84:433–435
Schaer M (1989) General principles of fluid therapy in small animal medicine. Vet Clin N Am Sm Anim Pract 19:203–213
Schoenherr W, Jewell J (1999) Effect of conjugated linoleic acid on body composition of mature obese Beagles. FASEB J 13:A262
Schneeman BO (1994) Carbohydrates: significance for energy balance and gastrointestinal function. J Nutr 124(Suppl 9):1747S-1753S
Schoenmakers I, Hazewinkel HAW, van den Brom WE (1999) Excessive Ca and P intake during early maturation in dogs alters Ca and P balance without long-term effects after dietary normalization. J Nutr 129:1068–1074
Schweigert FJ (1998) Metabolism of carotenoids in mammals. In: Liaaen-Jensen S, Pfander H (eds) Carotenoids (Britton G. Birkhäuser Verlag, Boston, pp 249–284
Schweigert FJ, Raila J, Wichert B, Kienzle E (2002) Cats absorb β-carotene, but it is not converted to vitamin A. J Nutr 132:1610S-1612S
Silvio J, Harmon DL, Gross KL, McLeod KR (2000) Influence of fiber fermentability on nutrient digestion in the dog. Nutrition 16:289–295
Sinclair AJ, Slattery W, McLean JG, Monger EA (1981) Essential fatty acid deficiency and evidence for arachidonate synthesis in the cat. Br J Nutr 46:93–96
Singh P, Banton S, Bosch G, Hendriks WH, Shoveller AK (2024) Beyond the bowl: understanding amino acid requirements and digestibility to improve protein quality metrics for dog and cat foods. Adv Exp Med Biol 1446:99–134. https://doi.org/10.1007/978-3-031-54192-6_5
Sparkes AH, Cannon M, Church D, Fleeman L, Harvey A, Hoenig M, Peterson ME, Reusch CE, Taylor S, Rosenberg D, ISFM (2015) ISFM consensus guidelines on the practical management of diabetes mellitus in cats. J Feline Med Surg 17:235–250
Summers SC, Stockman J, Larsen JA, Zhang L, Rodriguez AS (2020) Evaluation of phosphorus, calcium, and magnesium content in commercially available foods formulated for healthy cats. J Vet Intern Med 34:266–273
Supplee GC, Bellis B (1922) The coper content of cow’s milk. J Dairy Sci 5:455–467
Tôrres CL, Hickenbottom SJ, Quinton R. Rogers QR (2003) Palatability affects the percentage of metabolizable energy as protein selected by adult beagles. J Nutr 133:3516–3522
Trevizan L, de Mello KA, Bigley KE, Anderson WH, Waldron MK, Bauer JE (2010) Effects of dietary medium-chain triglycerides on plasma lipids and lipoprotein distribution and food aversion in cats. Am J Vet Res 71:435–440
Ugawa T, Kurihara K (1993) Large enhancement of canine taste responses to amino acids by salts. Am J Physiol 264:R1071-1076
Van Kruiningen HJ, Wojan LD, Stake PE, Lord PF (1987) The influence of diet and feeding frequency on gastric function in the dog. J Am Anim Hosp Assoc 23:146–153
Vendramini THA, Amaral AR, Pedrinelli V, Zafalon RVA, Rodrigues RBA, Brunetto MA (2020) Neutering in dogs and cats: current scientific evidence and importance of adequate nutritional management. Nutr Res Rev 33:134–144
Verbrugghe A, Bakovic M (2013) Peculiarities of one-carbon metabolism in the strict carnivorous cat and the role in feline hepatic lipidosis. Nutrients 5:2811–2835
Verbrugghe A, Hesta M (2017) Cats and carbohydrates: the carnivore fantasy? Vet Sci 4:55
Vhile SG, Skrede A, Ahlstrøm Ø, Hove K (2005) Comparative apparent total tract digestibility of major nutrients and amino acids in dogs (Canis familiaris), blue foxes (Alopex lagopus) and mink (Mustela vison). Anim Sci 81:141–148
Vinay P, Lemieux G, Gougoux A, Halperin M (1986) Regulation of glutamine metabolism in dog kidney in vivo. Kidney Int 29:68–79
Vuorinen A, Bailey-Hall E, Karagiannis A, Yu S, Roos F, Sylvester E, Wilson J, Irina Dahms I (2020) Safety of algal oil containing EPA and DHA in cats during gestation, lactation and growth. J Anim Physiol Anim Nutr (berl). 104:1509–1523
Wannemacher RW, McCoy JR (1966) Determination of optimal dietary protein requirements of young and old dogs. J Nutr 88:66–74
Washizu, T, Ishida T, Washizu M, Tomoda I, Kaneko JJ (1994) Changes in bile acid composition of serum and gallbladder bile in bile duct ligated dogs. J Vet Med Sci 56:299–303
Washizu T, Tanaka A, Sako T, Washizu M, Arai T (1999) Comparison of the activities of enzymes related to glycolysis and gluconeogenesis in the liver of dogs and cats. Res Vet Sci 67:205–206
Watford M (1985) Gluconeogenesis in the chicken: regulation of phosphoenolpyruvate carboxykinase gene expression. Fed Proc 44:2469–2074
Weber FL Jr, Maddrey WC, Walser M (1977) Amino acid metabolism of dog jejunum before and during absorption of keto analogues. Am J Physiol 232:E263-269
Weber FL Jr, Veach GL (1979) The importance of the small intestine in gut ammonium production in the fasting dog. Gastroenterology 77:235–240
Weber FL Jr, Friedman DW, Fresard KM (1988) Ammonia production from intraluminal amino acids in canine jejunum. Am J Physiol 254:G264-268
Weber MP, Stambouli F, Martin LJ, Dumon HJ, Biourge VC, Nguyen PG (2002) Influence of age and body size on gastrointestinal transit time of radioopaque markers in healthy dogs. Am J Vet Res 63:677–682
Weber MP, Martin LJ, Biourge VC, Nguyen PG, Dumon HJ (2003) Influence of age and body size on orocecal transit time as assessed by use of the sulfasalzine method in healthy dogs. Am J Vet Res 64:1105–1109
Wester TJ, Weidgraaf K, Hekman M, Ugarte CE, Forsyth SF, Tavendale MH (2015) Amino acid oxidation increases with dietary protein content in adult neutered male cats as measured using [1-13C]leucine and [15N2]urea. J Nutr 145:2471–2478
White TD, Boudreau JC (1975) Taste preferences of the cat for neurophysiologically active compounds. Physiol Psychol 3:405–410
Widdowson EM (1985) Development of the digestive system: comparative animal studies. Am J Clin Nutr 41(Suppl):384–390
Wildgrube HJ, Stockhausen H, Petri J, Füssel U, Lauer H (1986) Naturally occurring conjugated bile acids, measured by highperformance liquid chromatography, in human, dog, and rabbit bile. J Chromatogr 353:207–213
Williams CC, Cummins KA, Hayek MG, Davenport GM (2001) Effects of dietary protein on whole-body protein turnover and endocrine function in young-adult and aging dogs. J Anim Sci 79:3128–3136
Wolf LGG, Mehlman MA (1972) Subcellular distribution of pyruvate carboxylase and phosphoenolpyruvate carboxykinase in dog liver and kidney. Proc Soc Exp Biol Med 141:532–535
Woods JE, Besch EL (1971) Dissipation of body heat in dogs in a controlled environment. Physiologist 14:252
Wu G (1998) Amino acid metabolism in the small intestine. Trends Comp Biochem Physiol 4:39–74
Wu G (2009) Amino acids: metabolism, functions, and nutrition. Amino Acids 37:1–17 Wu G (2016) Dietary protein intake and human health. Food Funct 7:1251–1265
Wu G (2018) Principles of animal nutrition. CRC Press, Boca Raton, Florida
Wu G (2020a) Metabolism and functions of amino acids in sense organs. Adv Exp Med Biol 1265:201–217
Wu G (2020b) Important roles of dietary taurine, creatine, carnosine, anserine and 4-hydroxyproline in human nutrition and health. Amino Acids 52:329–360
Wu G (2022) Amino acids: biochemistry and nutrition, 2nd edn. CRC Press, Boca Raton, Florida
Wu G, Morris SM Jr (1998) Arginine metabolism: nitric oxide and beyond. Biochem J 336:1–17
Wu G, Li P (2022) The “ideal protein” concept is not ideal in animal nutrition. Exp Biol Med 247:1191–1201
Wu G, Borbolla AG, Knabe DA (1994) The uptake of glutamine and release of arginine, citrulline and proline by the small intestine of developing pigs. J Nutr 124:2437–2444
Wu G, Bazer FW, Satterfield MC, Li XL, Wang XQ, Johnson GA, Burghardt RC, Dai ZL, Wang JJ, Wu ZL (2013) Impacts of arginine nutrition on embryonic and fetal development in mammals. Amino Acids 45:241–256
Wu G, Cross HR, Gehring KB, Savell JW, Arnold AN, McNeill SH (2016a) Composition of free and peptide-bound amino acids in beef chuck, loin, and round cuts. J Anim Sci 94:2603–2613
Wu ZL, Hou YQ, Hu SD, Bazer FW, Meininger CJ, McNeal CJ, Wu G (2016b) Catabolism and safety of supplemental L-arginine in animals. Amino Acids 48:1541–1552
Xenoulis PG, Palculict B, Allenspach K, Steiner JM, Van House AM, Suchodolski JS (2008) Molecular-phylogenetic characterization of microbial communities imbalances in the small intestine of dogs with inflammatory bowel disease. FEMS Microbiol Ecol 66:579–589
Yu YM, Wagner DA, Tredget EE, Walaszewski JA, Burke JF, Young VR (1990) Quantitative role of splanchnic region in leucine metabolism: L-[1-13C,15N]leucine and substrate balance studies. Am J Physiol 259:E36-51
Yu YM, Burke JF, Tompkins RG, Martin R, Young VR (1996) Quantitative aspects of interorgan relationships among arginine and citrulline metabolism. Am J Physiol 271:E1098-1109
Yu S, Rogers QR, Morris JG (1997) Absence of a salt (NaCl) preference or appetite in sodium-replete or depleted kittens. Appetite 29:1–10
Yu S, Rogers QR, Morris JG (2001) Effect of low levels of dietary tyrosine on the hair colour of cats. J Small Anim Pract 42:176–180
Zafalon RVA, Risolia LW, Pedrinelli V, Vendramini THA, Rodrigues RBA, Amaral AR, Kogika MM, Brunetto MA (2020a) Vitamin D metabolism in dogs and cats and its relation to diseases not associated with bone metabolism. J Anim Physiol Anim Nutr 104:322–342
Zafalon RVA, Risolia LW, Vendramini THA, Ayres Rodrigues RB, Pedrinelli V, Teixeira FA et al (2020b) Nutritional inadequacies in commercial vegan foods for dogs and cats. PLoS ONE 15:e0227046
Zaghini G, Biagi G (2005) Nutritional peculiarities and diet palatability in the cat. Vet Res Commun 29(Suppl 2):39–44
Zhai H, Adeola O (2011) Apparent and standardized ileal digestibilities of amino acids for pigs fed corn-and soybean meal-based diets at varying crude protein levels. J Anim Sci 89:3626–3633
Zhang Q, Hou YQ, Bazer FW, He WL, Posey EA, Wu G (2021) Amino acids in swine nutrition and production. Adv Exp Med Biol 1285:81–107
Zoran DL (2010) Obesity in dogs and cats: a metabolic and endocrine disorder. Vet Clin North Am Small Anim Pract 40:221–239
Zoran DL (2023) Cat (Felis catus), essential nutrients. In: Phillips CJC (ed) Encyclopedia of Animal Nutrition, 2nd edition. CABI, Wallingford, pp 100–102








.jpg&w=3840&q=75)