Tick vaccine development
Published: May 28, 2014
By: Umair Ahsan, Haroon Rashid Chaudhry (University College of Veterinary and Animal Sciences, The Islamia University of Bahawalpur, Pakistan)
Introduction
Blood-feeding parasites have been reported to be the vectors of broad spectrum of disease agents since a long history. Ticks are one of those blood-sucking arthropods, responsible for a huge variety of heavy economic losses, caused by the direct effect of infestation, by injecting their toxins, through high morbidity and mortality, or by damaging the hides, and most importantly the reduction in production by lowering the performance of the animals (Book + Medical & Veterinary Entomology). de Castro, in 1997, reported that the annual global economic losses due to tick and tick borne diseases (TBDs) in cattle amount to between US$ 13.9 and US$ 18.7. The cost of losses in buffalo and small ruminants due to ticks and TBDs are still unavailable across the globe as well as in Pakistan’s livestock industry. Control of tick infestation has been a great challenge for the livestock industry of any country throughout the world. The conventional methods of controlling the tick infestation in animals include the pheromone assisted control, passive treatment, hormone assisted control, management, and eradication, all having certain limitations in their application (Medical & Veterinary Entomology). In addition to these control methods, use of acaricides for controlling the tick infestation has resulted in: the development of resistance in many species of ticks, showing partial success in controlling tick infestation, and the presence of residues in milk and meat (Willadsen and Kemp, 1988; Wikel, 1988; Nolan, 1990). The disadvantages of these control methods have necessitated the need to develop alternative tick control strategies. A report convincing the scientists, mammalian hosts can acquire immunization to tick feeding, has urged the researchers’ attention for achieving host protection from ticks (Tragger, 1939).
Principles of Host Immunity Development to Tick Infestation
The studies have made it clear that natural resistance to the ectoparasites is of two types either of innate or acquired origin (Allen, 1994; Barriga et al., 1995). Trager (1939) reported that a single infestation of a tick results in resistance in guinea pigs against the tick. Delayed hypersensitivity reactions have been reported in response to the ticks. The reactions occurring at the site of feeding are variable, ranging from cellular infiltration, inflammation and erythema to necrosis. One of the prominent features of lesion observed at the site of feeding is the accumulation of neutrophils, basophils, eosinophils, and lymphocytes (Allen, 1973; Bagnall, 1975; Brown and Knapp, 1981; Brown 1988). Signs such as an increased thickness, induration and necrosis may also be observed (Wikel et al., 1978). Cyclosporin-A (immunosuppressive) blocks the immediate (type-I) & delayed hypersensitivity (type-IV) reactions in response to salivary gland antigens of ticks, which confirms that cyclosporin-A sensitive cells (T-lymphocytes, mast cells and basophils) play a major role in the development of resistance to ticks (Giardin and Brossard, 1989); and enlargement of the local lymph nodes in resistant hosts also reveals that lymphocytes are the major immune cells involved in resistance response (Brown and Knapp, 1981).
Besides the cell mediated response to tick infestation, humoral response is also involved in the complex phenomenon of resistance to ticks. Partial immunity is transferred against various species of ticks in animals; it has been proved by conducting experiments (Trager, 1939; Robert and Kerr, 1976; Brossard, 1977). The titre of antibodies starts increasing by the end of first exposure of animal to ticks and it reaches to higher at second infestation (Bowessidjaou et al., 1977). The evidence to antibodies involvement can be confirmed by administering cyclophosphamide to immune animals before infestation; it specifically blocks the B-cells, although depletion of T-cells can be observed in some cases, however, its striking effect is only to block B-cells (Wikel and Allen, 1976a).
Sequential quantitative histological analysis of cellular infiltrate of tick feeding site demonstrates that neutrophils are dominant initially, followed by mononuclear cells, following primary infestation. While, after tertiary infestation, cellular infiltrate is characterized by massive degranulation of mast cells and basophils, basophils being the major effectors of resistance (Gill, 1984).
Candidate Vaccine Antigens
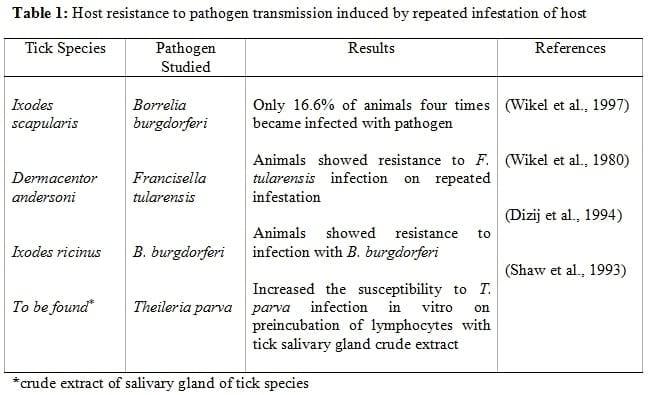
For the development of vaccine against any pathogenic organism, target antigens are identified at first. In the past, target antigens for vaccines against ticks were classified into two categories viz. exposed or concealed. Exposed antigens that directly come into contact with immune system of host when injected during blood feeding and concealed antigens those evade the immune system of host (Willadsen et al., 1988; Mulenga et al., 2000; Parizi et al., 2009). Attempts were made to use the salivary gland extract from the tick to immunize animals against blood sucking arthropods, but experiments showed that idea was impractical (Willadsen, 1980; Wikel, 1996). Continuous failure of exposed antigens in order to raise immunity against the ticks lead towards the usage of concealed antigens, this idea proposed that the control of ticks is possible using concealed antigens by raising the antibodies titre against some of the molecules such as hormones in the host which are ingested by the parasites during blood feeding(Galun, 1975). Later, it was observed that immunity raised by the concealed antigens is superior to that raised by the exposed antigens (Willadsen, 1987). Exposed antigens were considered to be evolved as a consequence of host immunity and could have reduced their antigenicity (Tellam et al., 1992) however, they are not supported by the experimental arguments. Some of the drawbacks such as failure to prevent feeding of ticks, damage to skin and hides, and also failure to prevention of transmission of TBDs, need to be addressed (Sahibi et al., 1997), although the commercial vaccine can be constituted using concealed antigens (Willadsen et al., 1995). Immunity against salivary gland components may interfere with events regulating tick attachment and feeding and disease transmission, as they are thought to be responsible for the establishment and regulation of tick feeding site as well as pathogen transmission (Ribeiro, 1987). Reports show that host resistance to pathogen transmission is induced by repeated infestation of host by ticks. A limited number of studies showing these results have been tabulated as under:
It can be concluded from the above findings that ticks do possess some of the components in their saliva which are involved in parasite establishment in the host, and acquired immunity against these factors can inhibit the pathogen transmission to hosts. Ticks are notorious as vectors and not as pests; therefore, the strategy of vaccine development should be based in order to prevent the parasite transmission irrespective of whether or not the vaccination results in tick mortality, the choice of target antigens based on arguments provided on both sides should be such that whether an antigen or a molecule is capable of preventing the host against both tick infestation and transmission of TBDs and not whether these antigens are exposed or concealed. The major criteria for the choice of antigens should be such that whether it is cross protective against other tick species. Trimnell et al., (2005) reported that
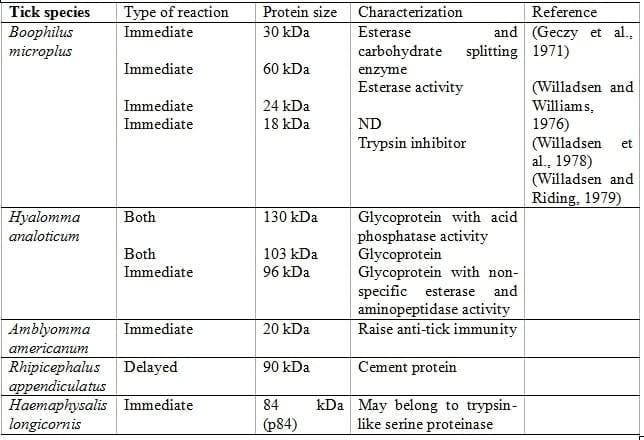
As a consequence, copious repair and defense mechanism are triggered, in response to tick inflicted injuries, involving haemostasis, thrombocytic activation and aggregation, and vasoconstriction (Ribeiro, 1987). Purpose of anti-tick host immune responses is to expel the obtrusive tick, and tick responds by secreting a number of pharmacologically active salivary components which counters the host haemostatic and immune effector mechanisms thus maintaining the continuous blood flow towards feeding site (Ribeiro, 1987), required by tick for longer periods between 10 to 14 days of blood feeding for repletion (Sonenshine, 1993). Host resistance to salivary gland coupled molecules is either naturally acquired by repetitive tick infestations or active immunization using crude tick extracts (Willadsen, 1980; Wikel, 1996). Characterization of natural antigens is based on their ability to: provoke skin hypersensitivity reaction in resistant host; respond to anti-tick immune serum or immune blots; alter the host immune response; and, inhibit the host blood coagulation mechanism (Table 2).
Table 2: Characterization of exposed antigen possessing different abilities
A) Antigens provoking skin hypersensitivity reaction
Target Identification
Various techniques can be used, in order to identify and characterize possible vaccine candidates, such as expression library immunization (ELI) (Almazán et al., 2003a; Almazán et al., 2003b), expressed sequence tags (ESTs) (Almazán et al., 2003b), signal sequence trapping (SST), cDNA libraries, micro-arrays, genome sequencing, proteomics, transcriptomics (Francischetti et al., 2005; Ribeiro et al., 2006), protein microarrays, and bioinformatics. An excellent global approach to identify vaccine antigens based on rapid screening of expressed genes has been observed, using ELI alongwith sequenced analysis of ESTs, which allows antigen identification without incorporating the available criteria to direct the selection of potential vaccine antigen candidates, resulting in the detection of novel and unexpected antigens (Almazán et al., 2003a; Almazán et al., 2003b). Although, it proved to be a better method yet there were the questions such as experimental immunization and challenge tick infestation of a huge quantity of laboratory animals are arduous, costly, intricate to standardize, and major problem being the host specificity of ticks that other species especially Boophilus spp. may require large animal hosts for infestation and subsequent feeding, leading towards complexity. Using these methods, a number of genes have been sequenced, but their functions are vague. All these demerits have been solved by RNA interference (RNAi), providing an imminent into gene function, and reducing animal challenge experimentation, thereby becoming an increasingly powerful post-transcriptional gene silencing technique (Kuwabara ans Coulson, 2000; Hannon, 2002). The process is ATP-dependent generation of small ≈21-25 nucleotide, short interfering RNA (siRNA) molecules from double stranded RNA (dsRNA) using dicer ribonuclease which targets and destroys specific mRNAs facilitated by the construction of RNA-induced silencing complex (RISC) complementary to mRNAs, works as a guide and recruits a ribonuclease ultimately resulting in cleavage of specific mRNAs (Hammond et al., 2000; Bass, 2001; Plasterk, 2002). Later, it was hypothesized that ticks do possess the machinery that forms siRNAs from dsRNA, which targets specific mRNAs (Aljamali et al., 2003). Histamine binding protein (HBP) of a female tick, Amblyomma americanum, was studied using RNAi. In this study, HBP dsRNA was constructed using RNA polymerases in vitro transcription, from A. americanum HBP dsRNA and 1010 dsRNA molecules in buffer were injected in unfed female ticks with the help of micro injections. It decreased their daily weight gain and extended feeding time, levels of HBP transcript observed to be low in experimental group than in control group; all these indicated that dsRNA has the capability of travelling towards salivary glands from the site of injection.
In future, there are chances to use high throughput approaches because of ever increasing biological information and to boost the prevention of TBDs.
References:
- Tick-borne Diseases and Poverty, 2003
- Medical & Veterinary Entomology, 2002
- Willadsen P, Kemp DH., Vaccination with concealed antigens for tick control Parasitol Today. 1988;4:196-198
- Wikel SK., Immunological control of hematophagous arthropod vectors: utilizing novel antigens. Vet Parasitol. 1988;29:235-64
- Nolan, J., Acaricide resistance in single and multi-host ticks and strategies for control. Parasitology.1990;32, 145-153
- Trager W., Acquired immunity to ticks. J Entomol 1939;25(1):57-81
- Allen, J. R., Host resistance to ectoparasites. Revue Scientifique et technique 1994;116(1):66-70
- Barriga, O. O.; Da Silva, S. S.; Azevedo, J. S. Relationships and influences between Boophilus microplus characteristics in tick-naive or repeatedly infested cattle. Vet Parasitol 1995;56(1-3):225-238
- de Castro, J.J. Sustainable tick and tick-borne disease control in livestock improvement in developing countries. Vet Parasitol. 1997;71:77-97
- Allen, J. R., Tick resistance: Basophils in skin reactions of resistant guinea pigs. Int J Parasitol 1973;3:195-200
- Almazán C, Kocan KM, Bergman DK, Garcia-Garcia JC, Blouin EF, de la Fuente J. Identification of protective antigens for the control of Ixodes scapularis infestations using cDNA expression library immunization. Vaccine 2003; 21: 1492–501.
Related topics:
Authors:
The Islamia University of Bahawalpur, Pakistan
The Islamia University of Bahawalpur, Pakistan
Recommend
Comment
Share
Recommend
Reply
The Islamia University of Bahawalpur, Pakistan
31 de mayo de 2014
Please check this link also.
https://groups.yahoo.com/neo/groups/PVMA-PVMC/conversations/topics/4581?l=1
Recommend
Reply
The Islamia University of Bahawalpur, Pakistan
31 de mayo de 2014
This vaccine is not available in Pakistan. It is available in Australia and Cuba with the name "Tick Gard" and "Gavac", respectively. It is quite possible if these are failed now.
Recommend
Reply
Recommend
Reply

Would you like to discuss another topic? Create a new post to engage with experts in the community.






.jpg&w=3840&q=75)