Milk at high temperature
Milk Protein Behavior at High Temperature
Published: May 10, 2011
By: Ihsan Ullah, Muhammad Omar, Muhammad Ali, Akram Khan (Sarhad University), Muhammad Subhan Qureshi , Iftikhar Ahmed
ABSTRACT
The present research was conducted to determine effects of high temperature (120 °C) on milk proteins. In the first half of the experiment, the protein contents of the selected milk samples at room temperature were determined by Eko-Milk analyzer. Afterwards the milk samples were subjected for the temperature treatment in autoclave at 120 °C for 20 minutes and protein contents of the milk were determined by Eko-Milk analyzer. For protein characterization milk samples were run on the SDS PAGE which imparted the banding pattern for all the proteins i.e., αS1, αS2, β casein and κ-casein and whey proteins i.e.,α lactalbumin and β lactoglobulin in the milk at both room temperature and at high temperature (120 oC). SDS-PAGE profiles showed that the banding order in the SDS-PAGE picture at room temperature was very prominent and in well order while the SDS-PAGE picture at 120 °C showed the degenerated bands. The results indicated that high temperature caused declining effects on protein contents of the milk.
Key words: SDS-PAGE, αS1, αS2, β casein, κ-casein.
INTRODUCTION
The milk protein system is probably the best-characterized food protein system, and provides milk with an extremely wide variety of remarkable functionalities. Proteins are highly complex biopolymers and play important functions in food systems as thickening, water binding, emulsion and foaming agents. Milk contains total of 3.3% proteins. Milk proteins contain all ten (10) essential amino acids required by humans. There are two major categories of milk protein that are broadly defined by their chemical composition and physical properties [4]. The casein family contains phosphorus and will coagulate or precipitate at pH 4.6. The serum (whey) proteins do not contain phosphorus, and these proteins remain in solution in milk at pH 4.6.
High temperature treatments can cause interactions between casein and whey proteins that affect the functional but not the nutritional properties. For example, at high temperatures, β-lactoglobulin can form a layer over the casein micelle that prevents curd formation in cheese [7].
The whey proteins are more sensitive to heat than the caseins. Higher heat treatments may cause denaturation of β-lactoglobulin, which is an advantage in the production of some foods (yogurt) and ingredients because of the ability of the proteins to bind more water. Denaturation causes a change in the physical structure of proteins, but generally does not affect the amino acid composition and thus the nutritional properties. Severe heat treatments such as ultra high pasteurization may cause some damage to heat sensitive amino acids and slightly decrease the nutritional content of the milk. The whey protein α-lactalbumin, however, is very heat stable [6].
The heat treatment of milk during commercial processing operations results in a number of physicochemical changes in the milk constituents. Significant changes occurring upon heating milk above 60 °C include the denaturation of whey proteins, the interactions between the denatured whey proteins and the casein micelles, the conversion of soluble calcium, magnesium and phosphate to the colloidal state [9],[7], [8] . The level of these changes depends on both the temperature and length of heat treatment. Casein is the major protein component of milk which represents 75-80 % of all milk proteins Due to its complex composition, casein belongs to the group of phospho-glycoproteins [5]. Also, casein belongs to the group of heat-stable proteins, because it does not coagulate when subjected to a high heat treatment.
MATERIALS AND METHODS
Sample Collection
Sample of milk were collected from market (warsak road Peshawar), dairy farm (Peshawar University dairy farm) and tetra pack packages (Tarang, tera pack and Milk Pack, tera pack). About 100 mL of milk sample was taken from each sample were divided into five aliquots, Each aliquot was subjected to serial dilution to adjust the protein contents for analysis. After dilution the aliquots were subjected to respective treatment.
Treatment of Milk Samples
To determine the effects of high temperature on both quantity and quality of milk, the milk samples were autoclaved at120 °C for 15 minutes in the Molecular Genetics Laboratory of the Institute of Biotechnology and Genetic Engineering, N.W.F.P Agricultural University Peshawar.
Milk Sample Analysis
To analyze the effects heating on milk constituents the following analytical procedures were applied.
Eko Milk Analysis for Protein Quantification
In this procedure 10% solution of acid cleaner EkoWeek was used as a periodical cleaning solution. 50 mL EkoWeek was added to glassware with 450 mL distilled water. Then the solution was poured into a labeled container. Both cleaning and alkaline solution were mixed together. The measuring mug was filled with 10 % solution of the acid cleaner EkoWeek (25°-40°C). The analyzer was put in cleaning mode and was set at 40 cycles and the OK button was pressed. When the CLEANING stage was over, CLEANING END was shown on the display. Plastic plug was taken (for Ekomilk Ultra Pro both plastic plugs) with the vinyl tube out of the holes. The plunger was ed instead of the plastic plug with the vinyl tube. The measuring mug was filled with clean and warm (but not hot) water (40°- 60°C). The plunger was Pull up and down several times. The mug was filled with clean and warm water was repeated 4-5 times. The measuring mug was removed and the plunger was taken out of syringe and the rubber plug was ed with the vinyl tube instead of the plunger.
SDS PAGE Analysis of the Total Protein Content
The protein composition of the treated milk samples was detected by the SDS-Poly Acrylamide Gel Electrophoresis (SDS-PAGE) performed according to the methods previously described [10]. Tubes containing milk proteins samples were placed in a boiling water bath for 3-4 minutes. 10μL proteins were loaded in each lane of each gel. The Electrophoresis apparatus was set at 20 mA of current at constant voltage 100 volt until the dye(Commassie Blue dye) was approximately 0.5 cm from the end of the gel. The gel was removed and placed in a small plastic container Commassie Blue stain and left for 90 min for staining. The gel was washed 3 times for 5 minutes in 200 mL of double distilled water. Then the gel covered with destaining solution the destaining solution was kept changing every few hours until the protein bands were visible. The gel was observed and photographed.
RESULTS
The present investigation deal with The effects of high temperature (120 °C) on milk proteins to compare the result and the investigation were caused out in two stages. In the first half instance protein contents of the selected milk samples were dermine room temperature using Eko-Milk analyzer. The result ae shown in table-1.
In the second half the milk samples were first subjected for the temperature treatment in autoclave at 120°C for 20 minutes then the protein contents of the milk were determined by Eko-Milk analyzer (1).
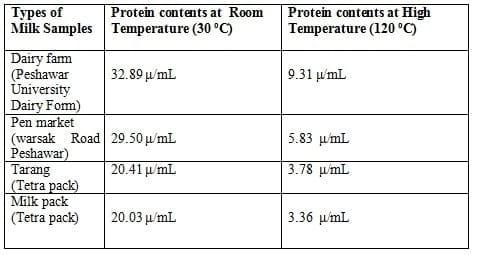
Table 1: Different protein contents of milk at respective temperatures
Comparing of the results obtained from Eko-milk analysis shown in Fig-1 that the all the protein contents in the milk were drifted to extreme declination. Protein contents of the milk before heat treatment were higher (Table 1; Fig 1) but after heat treatment at different temperature ( using auto clave ) imparted negative effects i.e. the decrease in the protein contents were (Table 1; Fig 1).
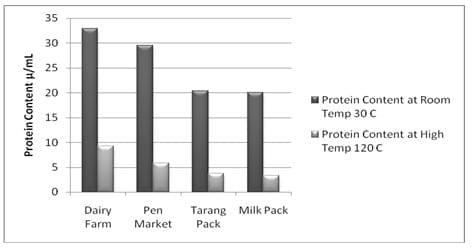
Fig 1: Effect of Temperature on protein contents of milk samples at respective temperatures.
Afterward the milk samples were defatted to prepare for the SDS-PAGE. The samples were run on the SDS PAGE which imparted banding pattern for all the proteins in the milk at room temperature. The banding position in the SDS PAGE profile at room temperature( 30 oC) showed that the quantities of αS1, αS2, β casein and κ-casein were high enough while the bands of whey proteins i.e., α lactalbumin and β lactoglobulin were also stronger.
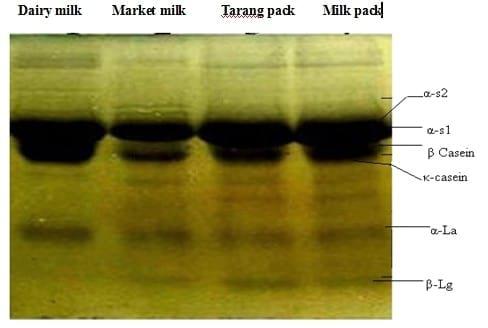
Fig 2: SDS PAGE of different milk samples at room temperature.
Similarly the autoclaved milk samples were run on SDS-PAGE to get the appropriate banding order of the milk proteins after the heat treatment at 30 oC. The results showed that the protein contents of the milk were completely degenerated and there were defused bands in the bottom of the SDS-PAGE ( Fig 2).
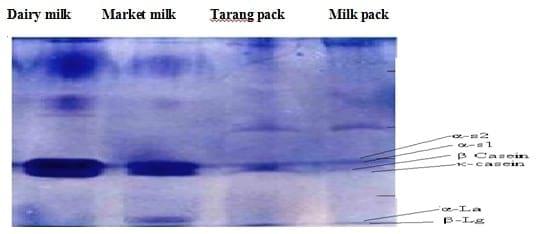
Fig 2.1:SDS PAGE of different milk samples at 120 °C temperature.
The two SDS-PAGE profiles showed that the banding order in the SDS-PAGE picture (Fig 2.1) at room temperature was very prominent and in well order while the SDS-PAGE picture at 120 °C showed (Fig 2.1) degenerated bands. The results indicate that high temperature caused declining effects on protein contents of the milk.
DISCUSSION
Results obtained from Eko-Milk analyzer at room temperature (30 oC) and high temperature (120 oC) treatments of milk samples conferred that high temperature 120 °C caused significant differences in protein content of the milk. The effect of heating on the protein contents of the milk in this study showed that when temperature was increased form room temperature (30 oC) to 120 °C the protein concentration showed a great declination in the concentration of the milk proteins. This aptitude was due to the break down of the polypeptide chains forming whey protein and the micelles of casein. [11] observed similar behavior of milk proteins at the prescribed range of temperatures. At room temperature whey proteins were aggregated by week Vander wall's forces and strong covalent bonds but these aggregates were ruptured at high temperatures. The large polypeptide was either converted to small polypeptide chains of amino acids at higher temperatures (120 oC) [3]. In the case of caseins, at room temperature there were strong casein micelles but at high temperature the micelles were completely dispersed [2].
SDS PAGE profile showed that at room temperature the quantities of αS1 and β caseins were very high as shown in (Fig 2) while autoclaving of milk samples at 120 oC for 20 min showed significant decrease in the quantities of αS1, αS2, β caseins, κ-caseins and the location of the bands shifted to the bottom at SDS-PAGE profile. Similar behivor was exhibited by whey proteins i.e., α-lactalbumin and β-lactoglobulin at high temperature. [1] studied similar banding pattern in SDS GAGE profile when the samples were heated at high temperatures.
SDS PAGE profile showed that at room temperature the quantities of αS1 and β caseins were very high as shown in (Fig 2) while autoclaving of milk samples at 120 oC for 20 min showed significant decrease in the quantities of αS1, αS2, β caseins, κ-caseins and the location of the bands shifted to the bottom at SDS-PAGE profile. Similar behivor was exhibited by whey proteins i.e., α-lactalbumin and β-lactoglobulin at high temperature. [1] studied similar banding pattern in SDS GAGE profile when the samples were heated at high temperatures.
SDS-PAGE analysis carried out by[10]clearly pointed out that the chemical complexes between whey proteins and casein were formed when the milk was subjected to autoclave at 120 °C. At higher temperature, high molecular weight protein molecules were disappeared almost completely and had been observed as a diffused zone.
CONCLUSION AND RECOMMENDATIONS
SDS-PAGE profiles revealed that the banding position in the SDS-PAGE profile at room temperature was very prominent and well ordered where as SDS-PAGE picture at 120 °C showed defused bands. The results obtained indicate that high temperature causes degenerative effects on protein contents of the milk. So we can suggest the following points must be practiced during milk usage.
- Fresh milk may be used to get maximum nutritional value.
- Milk should not be over heated because all the important protein contents are degenerated at high temperature.
- Milk should be moderately heated to destroy micro organisms which spoil milk.
ACKNOWLEDGMENT
We express our genuine thanks to the Department of Biotechnology, Sarhad University of Science and Information Technology, Pakistan for providing financial support and Biotechnology Lab. at Institute of Biotechnology and Genetic Engineering (IBGE), NWFP, Agricultural University Peshawar, Pakistan for providing research facilities.
References
1. Alakali, J.S., T.M. Okonkwo and S.A. Umoru, ( 2007). Effect of thermization on shelf stability of yoghurt. Ejeafche, Vol. 6, 1957-1964
2. Allmere, T.,A. Andrén, M. Lindersson and L. Björck, ( 1998) Studies on rheological properties of stirred milk gels made from milk with defined genetic variants of α-caseins,κ-casein and β-lactoglobulin. Inter Dairy J, Vol. 8, 899-905.
3. Bessie B. C., F.C. Jane, S. Beatrice, and F.M. Agnes. (1950). The effect of heat treatment on the nutritive value of milk proteins. Biochem. J; Vol.305, 51-58.
4. Evans,E. J and H. A. Butts. (2000). Studies on the heat inactivation of lysine in soybean oil meal J. Biol. Chem, 175: 15-22.
5. Fox, P. F. and P.L.H. McSweeney. (1998). Milk proteins. In Dairy Chem and Biochem, Vol. 23,230-239.
6. Kiyoshi, T., I. Teru, M. Yasumichi, M. Tetsuya, Y. Toshihiro, D. Toyohiko, I. Satoru, K. Tomihisa, Y. Kaoru and W. Ryozo. (1995). Heat Effect on the Taste of Milk Studied Using a Taste Sensor. Jpn. J. Appl. Phys, Vol. 34,6287-6291.
7. Macej, O., S. Jovanovic and J. D. Djurdjevic. (2002). The influence of high temperature on milk Proteins. Chem. Industry, Vol. 56,123-132.
8. Macej, O., S. Jovanovic, S. Seratlic and M. Barac. .(2004). Production of fresh chese with milk protein coagregates. Biotechnology in Animal Husbandry, Vol. 20, 119-131.
9. Singh, H. and A. Waungana. (2001). Influence of heat treatment of milk on cheesemaking properties. Int.Dairy J, Vol. 11, 543-551.
10. Snezana, J., B. Miroljub, M. Ognjen, V. Tanja and L. Caslav. (2007). SDS-PAGE Analysis of Soluble Proteins in Reconstituted Milk Exposed to Different Heat Treatments. Sensor, Vol. 7,371-383.
11. Simmons, B., A.T. Morgan, E.O.Weastandj. (2003). high temperature treatment changes milk properties J. Nutr, Vol. 58, 325-330.
Related topics:
Authors:
Livestock Management Department
Recommend
Comment
Share

Would you like to discuss another topic? Create a new post to engage with experts in the community.










