Influence of Precision Glycans on Layer Cecal Community
Glycans are ubiquitous complex polysaccharides present in all biological systems. Industry and the scientific community are becoming increasingly more aware of the importance of intestinal health in animal productivity and welfare. Novel strategies are emerging in developing intestinal health, targeting products that aim to control microbiome functions and alter the beneficial/pathogenic genera ratio in the gut. Precision glycans can be developed to improve intestinal health and functionality, and recent reports showed encouraging results. Here we present the effects of precision glycan-based intervention on the caecal intestinal microbiota in layers.
I. Introduction
II. Method
III. Results
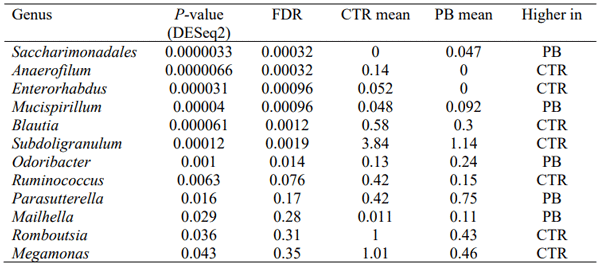
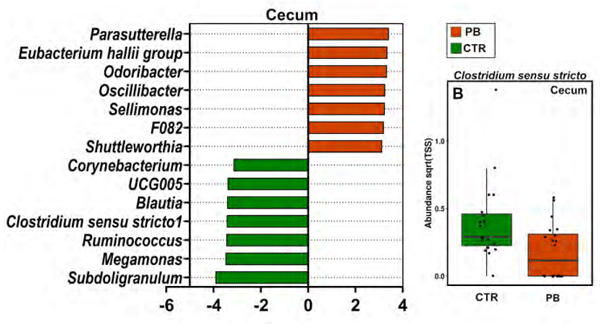
IV. Discussion
Allin KH, Tremaroli V, Caesar R, Jensen BAH, Damgaard MTF, Bahl MI, Licht TR, Hansen TH, Nielsen T, Dantoft TM, Linneberg A, Jorgensen T, Vestergaard H, Kristiansen K, Franks PW, consortium I-D, Hansen T, Backhed F & Pedersen O (2018) Diabetologia 61: 810-820.
Bush JR & Alfa MJ (2020) BMC Nutrition 6: 72.
Chen YJ, Wu H, Wu SD, Lu N, Wang YT, Liu HN, Dong L, Liu TT & Shen XZ (2018) Journal of Gastroenterology Hepatology 33: 1844-1852.
Coker JK, Moyne O, Rodionov DA & Zengler K (2021) Gut Microbes 13: 1-18.
Crost EH, Tailford LE, Monestier M, Swarbreck D, Henrissat B, Crossman LC & Juge N (2016) Gut Microbes 7: 302-312.
Foegeding NJ & Byndloss MX (2021) Cell Host Microbe 29: 851-853.
Herp S, Brugiroux S, Garzetti D, Ring D, Jochum LM, Beutler M, Eberl C, Hussain S, Walter S, Gerlach RG, Ruscheweyh HJ, Huson D, Sellin ME, Slack E, Hanson B, Loy A, Baines JF, Rausch P, Basic M, Bleich A, Berry D & Stecher B (2019) Cell Host Microbe 25: 681-694 e688.
Huang CB, Xiao L, Xing SC, Chen JY, Yang YW, Zhou Y, Chen W, Liang JB, Mi JD, Wang Y, Wu YB & Liao XD (2019) BMC Genomics 20: 770.
Ju T, Kong JY, Stothard P & Willing BP (2019) ISME Journal 13: 1520-1534.
Padovan A, Siboni N, Kaestli M, King WL, Seymour JR & Gibb K (2021) Marine Environmental Research 169: 105405.
Png CW, Linden SK, Gilshenan KS, Zoetendal EG, McSweeney CS, Sly LI, McGuckin MA & Florin TH (2010) American Journal of Gastroenterology 105: 2420-2428.
Ramirez S, Jacquier V, Walsh MC & Geremia JM (2021) Proceedings of the Australian Poultry Science Symposium 32: 30.
Robertson BR, O'Rourke JL, Neilan BA, Vandamme P, On SLW, Fox JG & Lee A (2005) International Journal of Systematic and Evolutionary Microbiology 55: 1199-1204.
Tannock GW (2021) Applied and Environmental Microbiology 87.
Vieira JRP, Rezende ATO, Fernandes MR & da Silva NA (2021) Advances in Rheumatology 61: 42.
Walsh MC, Jacquier V, Schyns G, Claypool J, Tamburini I, Blokker B & Geremia JM (2021) Poultry Science 100: 100800.
Zeng Q, Yang Z, Wang F, Li D, Liu Y, Wang D, Zhao X, Li Y, Wang Y, Feng X, Chen J, Li Y, Zheng Y, Kenney T, Gu H, Feng S, Li S, He Y, Xu X & Dai W (2021) Microbial Genomics 7.



United States







