Effectiveness of Bacillus strains as probiotics
Bacillus spp. Probiotic Strains as a Potential Tool for Limiting the Use of Antibiotics, and Improving the Growth and Health of Pigs and Chickens
The pressure to increasingly optimize the breeding of livestock monogastric animals resulted in antimicrobials often being misused in an attempt to improve growth performance and counteract diseases in these animals, leading to an increase in the problem of antibiotic resistance. To tackle this problem, the use of probiotics, also known as direct in-feed microbials (DFM), seems to be one of the most promising strategies. Among probiotics, the interest in Bacillus strains has been intensively increased in recent decades in pigs and poultry. The aim of the present review was to evaluate the effectiveness of Bacillus strains as probiotics and as a potential strategy for reducing the misuse of antibiotics in monogastric animals. Thus, the potential modes of action, and the effects on the performance and health of pigs (weaning pigs, lactation and gestation sows) and broilers are discussed. These searches yielded 131 articles (published before January 2021). The present review showed that Bacillus strains could favor growth in terms of the average daily gain (ADG) of post-weaning piglets and broilers, and reduce the incidence of post-weaning diarrhea in pigs by 30% and mortality in broilers by 6–8%. The benefits of Bacillus strains on these parameters showed results comparable to the benefit obtained by the use of antibiotics. Furthermore, the use of Bacillus strains gives promising results in enhancing the local adaptative immune response and in reducing the oxidative stress of broilers. Fewer data were available regarding the effect on sows. Discordant effects have been reported regarding the effect on body weight (BW) and feed intake while a number of studies have supported the hypothesis that feeding probiotics to sows could benefit their reproductive performance, namely the BW and ADG of the litters. Taken all the above-mentioned facts together, this review confirmed the effectiveness of Bacillus strains as probiotics in young pigs and broilers, favoring their health and contributing to a reduction in the misuse of direct in-feed antibiotics. The continuous development and research regarding probiotics will support a decrease in the misuse of antibiotics in livestock production in order to endorse a more sustainable rearing system in the near future.
Keywords: antibiotics, Bacillus, gut health, pig, probiotics, broiler.
INTRODUCTION
MODE OF ACTION OF Bacillus spp. PROBIOTIC STRAINS
Direct Effect on Pathogens
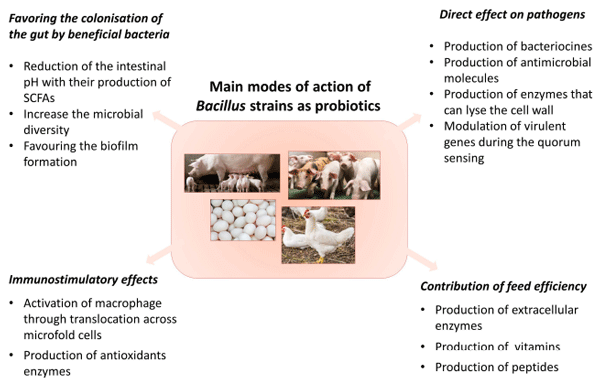
Favoring the Colonization of the Gut by Beneficial Bacteria
Immunostimulatory Effects
Contribution of Feed Efficiency
APPLICATION OF Bacillus spp. PROBIOTIC STRAINS IN LIVESTOCK MONOGASTRIC ANIMALS
Research Analysis Approach
Application of Bacillus Strains to Post-weaning Piglets
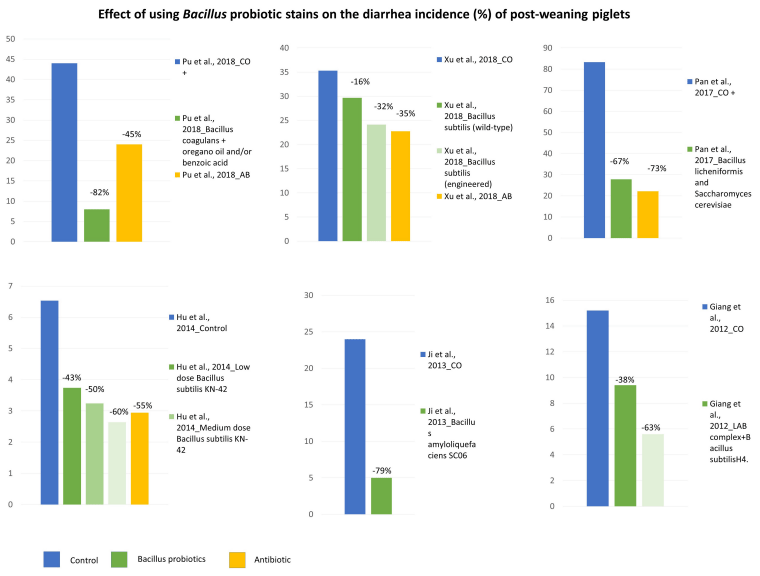
Application of Bacillus Probiotic Strains to Control Post-weaning Diarrhea
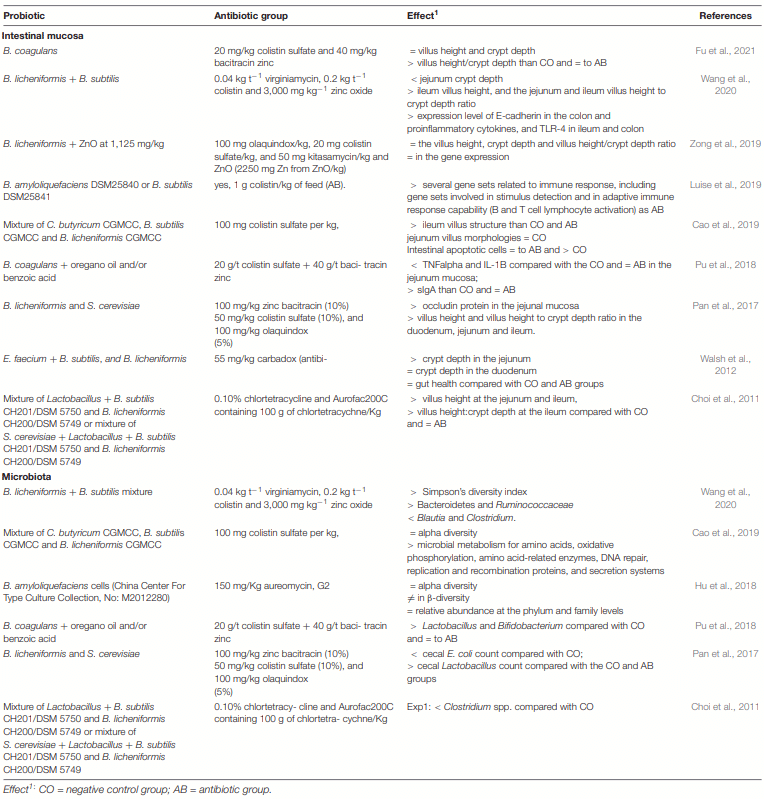
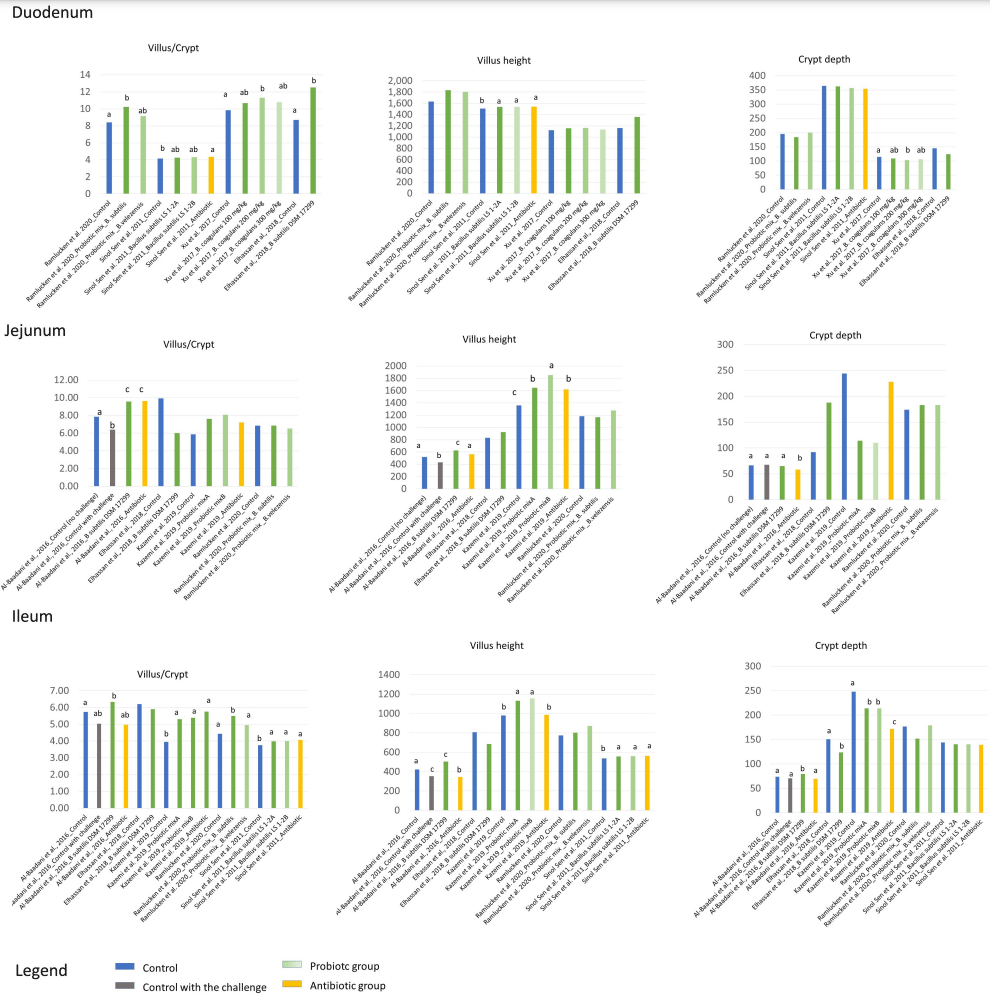
Application of Bacillus Probiotic Strains to Improve the Gut Health of Piglets
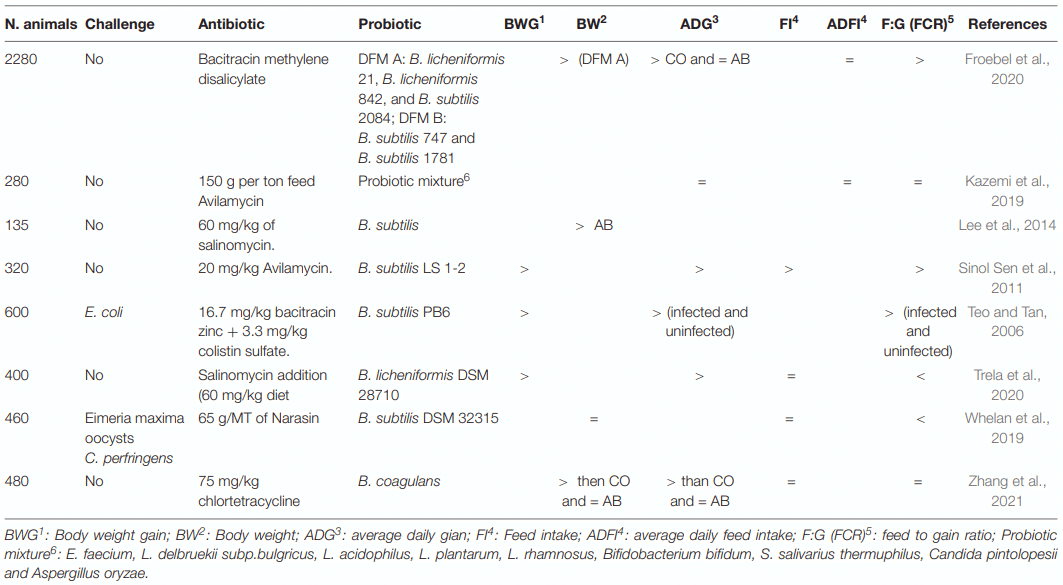
Application of Bacillus Probiotic Strains to Improve the Growth Performance and Feed Efficiency of Piglets
Application of Bacillus Probiotic Strains in Sows
Application of Bacillus Probiotic Strains for Improving Sow Body Weight and Fertility
Application of Bacillus Probiotic Strains for Controlling the Intestinal Microbial Balance of Sows
Application of Bacillus Probiotic Strains for Controlling Sow Oxidative Stress and Immunity Parameters
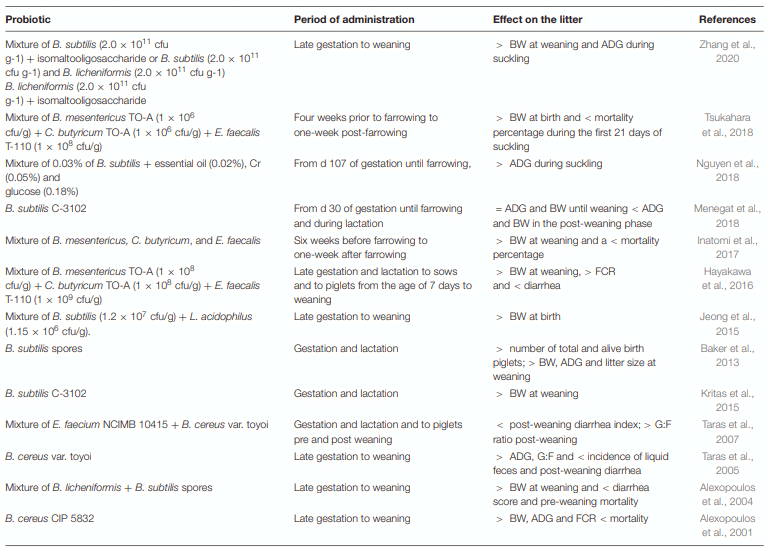
Application of Bacillus Probiotic Strains for Improving Colostrum Milk Quality and Litter Performance
Application of Bacillus Probiotic Strains to Broilers
Application of Bacillus Probiotic Strains to Improve the Health and Survival of Broilers
Effect of Bacillus Probiotic Strains on Gut Health, the Microbiota and Immune Functions of Broilers
Application of Bacillus Probiotic Strains to Improve Growth Performance and the Feed Efficiency of Broilers
CONCLUSION
AUTHOR CONTRIBUTIONS
SUPPLEMENTARY MATERIAL
Abdel-Moneim, A. E., Selim, D. A., Basuony, H. A., Sabic, E. M., Saleh, A. A., and Ebeid, T. A. (2020). Effect of dietary supplementation of Bacillus subtilis spores on growth performance, oxidative status, and digestive enzyme activities in Japanese quail birds. Trop. Anim. Health Prod. 52, 671–680. doi: 10.1007/ s11250-019-02055-1
Abriouel, H., Franz, C. M., Omar, N., and Ben Galvez, A. (2011). Diversity and applications of Bacillus bacteriocins. FEMS Microbiol. Rev. 35, 201–232.
Abudabos, A. M., Alyemni, A. H., Dafalla, Y. M., and Khan, R. U. (2017). Effect of organic acid blend and Bacillus subtilis alone or in combination on growth traits, blood biochemical and antioxidant status in broilers exposed to Salmonella typhimurium challenge during the starter phase. J. App. Anim. Reserc. 45, 538–542. doi: 10.1080/09712119.2016.1219665
Agyare, C., Boamah, V. E., Zumbi, C. N., and Osei, F. B. (2018). “Antimicrobial resistance - a global threat,” in Antibiotic Use in Poultry Production and Its Effects on Bacterial Resistance, ed. Y. Kumar (London: Intech Open), 1–20. doi: 10.5772/intechopen.79371
Aidara-Kane, A., Angulo, F. J., Conly, J. M., Minato, Y., Silbergeld, E. K., McEwen, S. A., et al. (2018). World Health Organization (WHO) guidelines on use of medically important antimicrobials in food-producing animals. Antimicrob. Resist. Infect. Control 7:7. doi: 10.1186/s13756-017-0294-9
Al-Baadani, H. H., Abudabos, A. M., Al-Mufarrej, S. I., and Alzawqari, M. (2016). Effects of dietary inclusion of probiotics, prebiotics and synbiotics on intestinal histological changes in challenged broiler chickens. S. Afr. J. Anim. Sci. 46, 157–165. doi: 10.4314/sajas.v46i2.6
Alexopoulos, C., Georgoulakis, I. E., Tzivara, A., Kritas, S. K., Siochu, A., and Kyriakis, S. C. (2004). Field Evaluation of the efficacy of a probiotic containing Bacillus licheniformis and Bacillus subtilis spores, on the health status and performance of sows and their litters. J. Anim. Physiol. Anim. Nutr. 88, 381– 3392. doi: 10.1111/j.1439-0396.2004.00492.x
Alexopoulos, C., Karagiannidis, A., Kritas, S. K., Boscos, C., Georgoulakis, I. E., and Kyriakis, S. C. (2001). Field evaluation of a bioregulator containing live Bacillus cereus spores on health status and performance of sows and their litters. J. Vet. Med. 48, 137–145. doi: 10.1046/j.1439-0442.2001.00342.x
Algammal, A. M., Hashem, H. R., Alfifi, K. J., Hetta, H. F., Sheraba, N. S., Ramadan, H., et al. (2021). atpD gene sequencing, multidrug resistance traits, virulence-determinants, and antimicrobial resistance genes of emerging XDR and MDR-Proteus mirabilis. Sci. Rep. 11:9476. doi: 10.1038/s41598-021- 88861-w
Algammal, A. M., Hetta, H. F., Elkelish, A., Alkhalifah, D. H. H., Hozzein, W. N., Batiha, G. E. S., et al. (2020). Methicillin-Resistant Staphylococcus aureus (MRSA): one health perspective approach to the bacterium epidemiology, virulence factors, antibiotic-resistance, and zoonotic impact. Infect. Drug Resist. 13, 3255–3265. doi: 10.2147/IDR.S272733
Altmeyer, S., Kröger, S., Vahjen, W., Zentek, J., and Scharek-Tedin, L. (2014). Impact of a probiotic Bacillus cereus strain on the jejunal epithelial barrier and on the NKG2D expressing immune cells during the weaning phase of piglets. Vet. Immunol. Immunopathol. 161, 57–65. doi: 10.1016/j.vetimm.2014.07.001
An, J., Zhu, W., Liu, Y., Zhang, X., Sun, L., Hong, P., et al. (2015). Purification and characterization of a novel bacteriocin CAMT2 produced by Bacillus amyloliquefaciens isolated from marine fish Epinephelus areolatus. Food Control. 51, 278–282. doi: 10.1016/j.foodcont.2014.11.038
Arnaouteli, S., Bamford, N. C., Stanley-Wall, N. R., and Kovács, ÁT. (2021). Bacillus subtilis biofilm formation and social interactions. Nat. Rev. Microbiol. 19, 600–614. doi: 10.1038/s41579-021-00540-9
Bai, K., Huang, Q., Zhang, J., He, J., Zhang, L., and Wang, T. (2017). Supplemental effects of probiotic Bacillus subtilis fmbJ on growth performance, antioxidant capacity, and meat quality of broiler chickens. Poult. Sci. 96, 74–82. doi: 10. 3382/ps/pew246
Bajagai, Y. S., Klieve, A. V., Dart, P. J., and Bryden, W. L. (2016). Probiotics in Animal Nutrition: Production, Impact and Regulation. Rome: FAO.
Baker, A. A., Davis, E., Spencer, J. D., Moser, R., and Rehberger, T. (2013). The effect of a Bacillus-based direct-fed microbial supplemented to sows on the gastrointestinal microbiota of their neonatal piglets. J. Anim. Sci. 91, 3390–3399. doi: 10.2527/jas.2012-5821
Barba-Vidal, E., Martín-Orúe, S. M., and Castillejos, L. (2019). Practical aspects of the use of probiotics in pig production: a review. Livest. Sci. 223, 84–96. doi: 10.2527/jas.2007-0459
Blajman, J. E., Olivero, C. A., Fusari, M. L., Zimmermann, J. A., Rossler, E., Berisvil, A. P., et al. (2017). Impact of lyophilized Lactobacillus salivarius DSPV 001P administration on growth performance, microbial translocation, and gastrointestinal microbiota of broilers reared under low ambient temperature. Res. Vet. Sci. 114, 388–394. doi: 10.1016/j.rvsc.2017.07.011
Blin, K., Shaw, S., Steinke, K., Villebro, R., Ziemert, N., Lee, S. Y., et al. (2019). antiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 47, W81–W87. doi: 10.1093/nar/gkz310
Cai, L., Indrakumar, S., Kiarie, E., and Kim, I. H. (2015). Effects of a multi-strain Bacillus species–based direct-fed microbial on growth performance, nutrient digestibility, blood profile, and gut health in nursery pigs fed corn–soybean meal–based diets. J. Anim. Sci. 93, 4336–4342. doi: 10.2527/jas.2015-9056
Cameron, A., and McAllister, T. A. (2019). Could probiotics be the panacea alternative to the use of antimicrobials in livestock diets? Benef. Microbes 10, 773–799. doi: 10.3920/BM2019.0059
Canning, P., Ruston, C. R., Madson, D., Bates, J., Skoland, K. J., Davenport, J., et al. (2017). Effect of direct-fed microbial Bacillus subtilis C-3102 on enteric health in nursery pigs after challenge with porcine epidemic diarrhea virus. J. Swine Health Prod. 25, 129–137.
Cao, G., Tao, F., Hu, Y., Li, Z., Zhang, Y., Deng, B., et al. (2019). Positive effects of a: Clostridium butyricum-based compound probiotic on growth performance, immune responses, intestinal morphology, hypothalamic neurotransmitters, and colonic microbiota in weaned piglets. Food Funct. 10, 2926–2934. doi: 10.1039/c8fo02370k
Chalvon-Demersay, T., Luise, D., Le, N., Tesseraud, S., Lambert, W., Bosi, P., et al. (2021). Functional amino acids in pigs and chickens: implication for gut health. Front. Vet. Sci. 8:663727. doi: 10.3389/fvets.2021.663727
Chaucheyras-Durand, F., and Durand, H. (2010). Probiotics in animal nutrition and health. Benef. Microbes 1, 3–9. doi: 10.3920/BM2008.1002
Checcucci, A., Trevisi, P., Luise, D., Modesto, M., Blasioli, S., Braschi, I., et al. (2020). Exploring the animal waste resistome: the spread of antimicrobial resistance genes through the use of livestock manure. Front. Microbiol. 11:1416. doi: 10.3389/fmicb.2020.01416
Chen, F., Gao, Y., Chen, X., Yu, Z., and Li, X. (2013). Quorum quenching enzymes and their application in degrading signal molecules to block quorum sensing-dependent infection. Int. J. Mol. Sci. 14, 17477–17500. doi: 10.3390/ ijms140917477
Choi, J. Y., Kim, J. S., Ingale, S. L., Kim, K. H., Shinde, P. L., Kwon, I. K., et al. (2011). Effect of potential multimicrobe probiotic product processed by high drying temperature and antibiotic on performance of weanling pigs. J. Anim. Sci. 89, 1795–1804. doi: 10.2527/jas.2009-2794
Ciurescu, G., Dumitru, M., Gheorghe, A., Untea, A. E., and Drãghici, R. (2020). Effect of Bacillus subtilis on growth performance, bone mineralization, and bacterial population of broilers fed with different protein sources. Poult. Sci. 99, 5960–5971. doi: 10.1016/j.psj.2020.08.075
Cladera-Olivera, F., Caron, G. R., and Brandelli, A. (2004). Bacteriocin like substance production by Bacillus licheniformis strain P40. Lett. Appl. Microbiol. 38, 251–256. doi: 10.1111/j.1472-765x.2004.01478.x Cutting, S. M. (2011). Bacillus probiotics. Food Microbiol. 28, 214–220. doi: 10. 1016/j.fm.2010.03.007
Dial, G. D., Marsh, W. E., Polson, D. D., and Vaillancourt, J.-P. (1992). “Reproductive failure: differential diagnosis,” in Diseases of Swine, eds A. D. Leman, B. E. Straw, S. Mengeling, W. L. S. D’Allaire, and D. J. Taylor (Iowa: Iowa State University Press), 88.
Ding, S., Yan, W., Ma, Y., and Fang, J. (2021). The impact of probiotics on gut health via alternation of immune status of monogastric animals. Anim. Nutr. 7, 24–30. doi: 10.1016/j.aninu.2020.11.004
Ding, H., Zhao, X., Ma, C., Gao, Q., Yin, Y., Kong, X., et al. (2021). Dietary supplementation with Bacillus subtilis DSM 32315 alters the intestinal microbiota and metabolites in weaned piglets. J. Appl. Microbiol. 130, 217–232. doi: 10.1111/jam.14767
Dong, Y. H., Gusti, A. R., Zhang, Q., Xu, J. L., and Zhang, L. H. (2002). Identification of quorum-quenching N-acyl homoserine lactonases from Bacillus species. Appl. Environ. Microbiol. 68, 1754–1759. doi: 10.1128/AEM. 68.4.1754-1759.2002
Duc, L. H., Hong, H. A., Fairweather, N., Ricca, E., and Cutting, S. M. (2003). Bacterial spores as vaccine vehicles. Infect. Immun. 71, 2810–2818.
Dumitru, M., Habeanu, M., Lefter, N. A., and Gheorghe, A. (2020). The effect of Bacillus licheniformis as direct-fed microbial product on growth performance, gastrointestinal disorders and microflora population in weaning piglets. Rom. Biotechnol. Lett. 25, 2060–2069.
EFSA Panel on Additives and Products or Substances used in Animal Feed [FEEDAP] (2014). Guidance on the assessment of the toxigenic potential of Bacillus species used in animal nutrition. EFSA J. 12:3665.
Elshaghabee, F. M. F., Rokana, N., Gulhane, R. D., Sharma, C., and Panwar, H. (2017). Bacillus as potential probiotics: status, concerns, and future perspectives. Front. Microbiol. 8:1490. doi: 10.3389/fmicb.2017.01490
European Commission (2020). Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions a Farm to Fork Strategy for a Fair, Healthy and Environmentally-Friendly Food System COM/2020/381 final. Available online at: https://eur-lex.europa.eu/legal-content/EN/TXT/ ?uri=CELEX:52020DC0381
European Union [EU] (2003). Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. Off. J. Eur. Union 50:29.
European Union [EU] (2007). Council Directive 2007/43/EC Laying down minimum rules for the protection of chickens kept for meat production. Off. J. Eur. Union 182, 19–28.
Eurostat (2022). Eurostat—Data Explorer. Available online at: https://appsso. eurostat.ec.europa.eu/nui/show.do?dataset=apro_mt_lspig&lang=en (accessed January 23, 2022).
FDA (2015). CPG Sec. 689.100 Direct-fed Microbial Products. Available online at: https://www.fda.gov/inspections-compliance-enforcement-and-criminalinvestigations/compliance-manuals.
Forte, C., Acuti, G., Manuali, E., Proietti, P. C., Pavone, S., Trabalza-Marinucci, M., et al. (2016). Effects of two different probiotics on microflora, morphology, and morphometry of gut in organic laying hens. Poult. Sci. 95, 2528–2535. doi: 10.3382/ps/pew164
Froebel, L. K., Jalukar, S., Lavergne, T. A., Coufal, C. D., and Duong, T. (2020). Administration of direct-fed Bacillus cultures and refined functional carbohydrates to broiler chickens improves growth performance and promotes positive shifts in gastrointestinal microbiota. J. Appl. Poult. Res. 29, 765–774.
Fu, R., Liang, C., Chen, D., Yan, H., Tian, G., Zheng, P., et al. (2021). Effects of dietary Bacillus coagulans and yeast hydrolysate supplementation on growth performance, immune response and intestinal barrier function in weaned piglets. J. Anim. Physiol. Anim. Nutr. (Berl). 105, 898–907. doi: 10.1111/jpn. 13529
Ghani, M., Ansari, A., Aman, A., Zohra, R. R., Siddiqui, N. N., and Qader, S. A. U. (2013). Isolation and characterization of different strains of Bacillus licheniformis for the production of commercially significant enzymes. Pak. J. Pharm. Sci. 26, 691–697.
Gong, L., Wang, B., Mei, X., Xu, H., Qin, Y., Li, W., et al. (2018). Effects of three probiotic Bacillus on growth performance, digestive enzyme activities, antioxidative capacity, serum immunity, and biochemical parameters in broilers. Anim. Sci. J. 89, 1561–1571. doi: 10.1111/asj.13089
Gresse, R., Chaucheyras-durand, F., Fleury, M. A., Van de Wiele, T., Forano, E., and Blanquet-diot, S. (2017). Gut microbiota dysbiosis in postweaning piglets: understanding the keys to health. Trends Microbiol. 25, 851–873. doi: 10.1016/ j.tim.2017.05.004
Gresse, R., Durand, F. C., Duniôlre, L., Blanquet-Diot, S., and Forano, E. (2019). Microbiota composition and functional profiling throughout the gastrointestinal tract of commercial weaning piglets. Microorganisms 7:343. doi: 10.3390/microorganisms7090343
Gu, X. L., Li, H., Song, Z. H., Ding, Y. N., He, X., and Fan, Z. Y. (2019a). Effects of isomaltooligos accharide and Bacillus supplementation on sow performance, serum metabolites, and serum and placental oxidative status. Anim. Reprod. Sci. 207, 52–60. doi: 10.1016/j.anireprosci.2019.05.015
Gu, X. L., Song, Z. H., Li, H., Wu, S., Wu, S. S., Ding, Y. N., et al. (2019b). Effects of dietary isomaltooligosaccharide and Bacillus spp. supplementation during perinatal period on lactational performance, blood metabolites, and milk composition of sows. J. Sci. Food Agric. 99, 5646–5653. doi: 10.1002/jsfa.9821
Gupta, M., Kaur, M., and Gupta, K. G. (1992). Lytic effect of Bacillus subtilis elastase on gram-positive and negative bacteria. Indian J. Exp. Biol. 30, 380–383.
Harwood, C. R., Mouillon, J. M., Pohl, S., and Arnau, J. (2018). Secondary metabolite production and the safety of industrially important members of the Bacillus subtilis group. FEMS Microbiol. Rev. 42, 721–738. doi: 10.1093/femsre/ fuy028
Hayakawa, T., Masuda, T., Kurosawa, D., and Tsukahara, T. (2016). Dietary administration of probiotics to sows and/or their neonates improves the reproductive performance, incidence of post-weaning diarrhea and histopathological parameters in the intestine of weaned piglets: beneficial effects of probiotics in pigs. Anim. Sci. J. 87, 1501–1510.
He, Y., Jinno, C., Kim, K., Wu, Z., Tan, B., Li, X., et al. (2020). Dietary Bacillus spp. enhanced growth and disease resistance of weaned pigs by modulating intestinal microbiota and systemic immunity. J. Anim. Sci. Biotechnol. 11:101. doi: 10.1186/s40104-020-00498-3
Hu, S., Cao, X., Wu, Y., Mei, X., Xu, H., Wang, Y., et al. (2018). Effects of probiotic Bacillus as an alternative of antibiotics on digestive enzymes activity and intestinal integrity of piglets. Front. Microbiol. 9:2427. doi: 10.3389/fmicb. 2018.02427
Hu, Y., Dun, Y., Li, S., Zhao, S., Peng, N., and Liang, Y. (2014). Effects of Bacillus subtilis KN-42 on growth performance, diarrhea and faecal bacterial flora of weaned piglets. Asian-Australas J. Anim. Sci. 27, 1131–1140. doi: 10.5713/ajas. 2013.13737
Huang, Q., Xu, X., Mao, Y. L., Huang, Y., Rajput, I. R., and Li, W. F. (2013). Effects of Bacillus subtilis B10 spores on viability and biological functions of murine macrophages. Anim. Sci. J. 84, 247–52. doi: 10.1111/j.1740-0929.2012.01064.x
Inatomi, T., Amatatsu, M., Romero-Pérez Gustavo, A., Inoue, R., and Tsukahara, T. (2017). Dietary probiotic compound improves reproductive performance of porcine epidemic diarrhea virus-infected sows reared in a Japanese commercial swine farm under vaccine control condition. Front. Immunol. 8:8. doi: 10.3389/ fimmu.2017.01877
Jansen, E. F., and Hirschmann, D. J. (1944). Subtilin, an antibacterial substance of Bacillus subtilis. Culturing condition and properties. Arch. Biochem. 4, 297–309.
Jeong, J., Kim, J., Lee, S., and Kim, I. (2015). Evaluation of Bacillus subtilis and Lactobacillus acidophilus probiotic supplementation on reproductive performance and noxious gas emission in sows. Ann. Anim. Sci. 15, 699–709. doi: 10.1515/aoas-2015-0018
Ji, J., Hu, S., Zheng, M., Du, W., Shang, Q., and Li, W. (2013). Bacillus amyloliquefaciens SC06 inhibits ETEC-induced pro-inflammatory responses by suppression of MAPK signaling pathways in IPEC-1 cells and diarrhea in weaned piglets. Livest. Sci. 158, 206–214. doi: 10.1016/j.livsci.2013.09.017
Jia, R., Sadiq, F. A., Liu, W., Cao, L., and Shen, Z. (2021). Protective effects of Bacillus subtilis ASAG 216 on growth performance, antioxidant capacity, gut microbiota and tissues residues of weaned piglets fed deoxynivalenol contaminated diets. Food Chem. Toxicol. 148:111962. doi: 10.1016/j.fct.2020. 111962
Joerger, R. D., and Ganguly, A. (2017). Current status of the preharvest application of pro- and prebiotics to farm animals to enhance the microbial safety of animal products. Microbiol Spectr. 5. doi: 10.1128/microbiolspec.PFS-0012-2016 [Epub ahead of print].
Johnson, C. T., Lupson, G. R., and Lawrence, K. E. (1994). The bovine placentome in bacterial and mycotic abortions. Vet. Rec. 134, 263–266. doi: 10.1136/vr.134. 11.263
Jørgensen, J. N., Laguna, J. S., Millán, C., Casabuena, O., and Gracia, M. I. (2016). Effects of a Bacillus-based probiotic and dietary energy content on the performance and nutrient digestibility of wean to finish pigs. Anim. Feed Sci. Technol. 221, 54–61.
Kazemi, S. A., Ahmadi, H., and Karimi Torshizi, M. A. (2019). Evaluating two multistrain probiotics on growth performance, intestinal morphology, lipid oxidation and ileal microflora in chickens. J. Anim. Physiol. Anim. Nutr. 103, 1399–1407. doi: 10.1111/jpn.13124
Kim, K., He, Y., Xiong, X., Ehrlich, A., Li, X., Raybould, H., et al. (2019). Dietary supplementation of Bacillus subtilis influenced intestinal health of weaned pigs experimentally infected with a pathogenic E. coli. J. Anim. Sci. Biotechnol. 10:52.
Kim, S. Y., Ohk, S. H., Bai, D. H., and Yu, J. H. (1999). Purification and properties of bacteriolytic enzymes from Bacillus licheniformis YS-1005 against Streptococcus mutans. Biosci. Biotechnol. Biochem. 63, 73–77. doi: 10.1271/bbb.63.73
Kimera, Z. I., Mgaya, F. X., Misinzo, G., Mshana, S. E., Moremi, N., and Matee, M. I. (2021). Multidrug-resistant, including extended-spectrum beta lactamaseproducing and quinolone-resistant, escherichia coli isolated from poultry and domestic pigs in dar es salaam, Tanzania. Antibiotics 10:406. doi: 10.3390/ antibiotics10040406
Koli, D., Kadam, M., Gole, M., Patil, A., Hajare, S., Yeskal, A., et al. (2018). Efficacy of Bacillus subtilis (Gallipro) supplementation in Clostridium perfringens challenged necrotic enteritis of broiler chicken. Indian J. Anim. Res. 52, 619– 622.
Konieczka, P., Nowicka, K., Madar, M., Taciak, M., and Smulikowska, S. (2018). Effects of pea extrusion and enzyme and probiotic supplementation on performance, microbiota activity and biofilm formation in the broiler gastrointestinal tract. Br. Poult. Sci. 59, 654–662. doi: 10.1080/00071668.2018. 1507017
Kordel, M., Schuller, F., and Sahl, H. G. (1989). Interaction of the pore formingpeptide antibiotics pep 5, nisin and subtilin with non-energized liposomes. FEBS Lett. 244, 99–102. doi: 10.1016/0014-5793(89)81171-8
Kritas, S. K., Marubashi, T., Filioussis, G., Petridou, E., Christodoulopoulos, G., Burriel, A. R., et al. (2015). Reproductive performance of sows was improved by administration of a sporing bacillary probiotic (Bacillus subtilis C-3102). J. Anim. Sci. 93, 405–413. doi: 10.2527/jas.2014-7651
Kunavue, N., and Lien, T. F. (2012). Effects of fulvic acid and probiotic on growth performance, nutrient digestibility, blood parameters and immunity of pigs. J Anim. Sci. Adv. 2, 711–721.
Lan, R. X., Lee, S. I., and Kim, I. H. (2016). Effects of multistrain probiotics on growth performance, nutrient digestibility, blood profiles, faecal microbial shedding, faecal score and noxious gas emission in weaning pigs. J. Anim. Physiol. Anim. Nutr. (Berl). 100, 1130–1138. doi: 10.1111/jpn.12501
Lee, K. W., Lee, S. H., Lillehoj, H. S., Li, G. X., Jang, S. I., Babu, U. S., et al. (2010). Effects of direct-fed microbials on growth performance, gut morphometry, and immune characteristics in broiler chickens. Poult. Sci. 89, 203–216. doi: 10.3382/ps.2009-00418
Lee, K. W., Lillehoj, H. S., Jang, S. I., and Lee, S. H. (2014). Effects of salinomycin and Bacillus subtilis on growth performance and immune responses in broiler chickens. Res. Vet. Sci. 97, 304–308. doi: 10.1016/j.rvsc.2014.07.021
Leman, A. D. (1990). Mate sows once 3-5 days after weaning. Int. Pigletter 10, 29–31.
Li, C. L., Wang, J., Zhang, H. J., Wu, S. G., Hui, Q. R., Yang, C. B., et al. (2019). Intestinal morphologic and microbiota responses to dietary Bacillus spp. in a broiler chicken model. Front. Physiol. 9:1968. doi: 10.3389/fphys.2018.01968
Li, H. H., Jiang, X. R., and Qiao, J. Y. (2021). Effect of dietary Bacillus subtilis on growth performance and serum biochemical and immune indexes in weaned piglets. J. Appl. Anim. Res. 49, 83–88. doi: 10.1080/09712119.2021.1877717
Li, Y., Li, X., Jia, D., Liu, J., Wang, J., Liu, A., et al. (2020). Complete genome sequence and antimicrobial activity of Bacillus velezensis JT3-1, a microbial germicide isolated from yak feces. 3 Biotech 10:231. doi: 10.1007/s13205-020- 02235-z
Lourenco, M. C., Kuritza, L. N., Westphal, P., Muniz, E., Pickler, L., and Santin, E. (2012). Effects of Bacillus subtilis in the dynamics of infiltration of immunological cells in the intestinal mucosa of chickens challenged with Salmonella Minnesota. Int. J. Poult. Sci. 11, 630–634. doi: 10.3923/ijps.2012.630. 634
Luise, D., Bertocchi, M., Motta, V., Salvarani, C., Bosi, P., Luppi, A., et al. (2019). Bacillus sp. probiotic supplementation diminish the Escherichia coli F4ac infection in susceptible weaned pigs by influencing the intestinal immune response, intestinal microbiota and blood metabolomics. J. Anim. Sci. Biotechnol. 10:74. doi: 10.1186/s40104-019-0380-3
Martirani, L., Varcamonti, M., Naclerio, G., and Felice, M. D. (2002). Purification and partial characterization of bacillocin 490, a novel bacteriocin produced by a thermophilic strain of Bacillus licheniformis. Microb. Cell Fact. 1:1. doi: 10.1186/1475-2859-1-1
Menegat, M. B., DeRouchey, J. M., Woodworth, J. C., Dritz, S. S., Tokach, M. D., and Goodband, R. D. (2019). Effects of Bacillus subtilis C-3102 on sow and progeny performance, fecal consistency, and fecal microbes during gestation, lactation, and nursery periods. J. Anim. Sci. 97, 3920–3937. doi: 10.1093/jas/ skz236
Menegat, M. B., Gourley, K. M., Braun, M. B., DeRouchey, J. M., Woodworth, J. C., Bryte, J., et al. (2018). Effects of a Bacillus-based probiotic on sow performance and on progeny growth performance, fecal consistency, and fecal microflora. Kans. Agric. Exp. Stn. Res. Rep. 4:9. doi: 10.4148/2378-5977.7652
Miller, M. B., and Bassler, B. L. (2001). Quorum sensing in bacteria. Annu. Rev. Microbiol. 55, 165–199. Mingmongkolchai, S., and Panbangred, W. (2018). Bacillus probiotics: an alternative to antibiotics for livestock production. J. Appl. Microbiol. 124, 1334–1346. doi: 10.1111/jam.13690
Mohammed, Y., Lee, B., Kang, Z., and Du, G. (2014). Development of a two-step cultivation strategy for the production of vitamin B12 by Bacillus megaterium. Microb. Cell Fact. 13:102. doi: 10.1186/s12934-014-0102-7
Neveling, D. P., and Dicks, L. M. T. (2021). Probiotics: an antibiotic replacement strategy for healthy broilers and productive rearing. Probiotics Antimicrob. Proteins 13, 1–11. doi: 10.1007/s12602-020-09640-z
Nguyen, D. H., Lan, R. X., and Kim, I. H. (2018). Bacillus subtilis, essential oil, chromium and glucose as sow pack alters the performance, immune and stress on pregnant sows and piglets. Indian J. Anim. Res. 52, 1174–1179.
Ohimain, E. I., and Ofongo, R. T. S. (2012). The effect of probiotic and Prebiotic feed supplementation on chicken health and gut microflora: a review. Int. J. Anim. Vet. Adv. 4, 135–143. doi: 10.1128/AEM.00600-20
Ongena, M., Jacques, P., Touré, Y., Destain, J., Jabrane, A., and Thonart, P. (2005). Involvement of fengycin-type lipopeptides in the multifaceted biocontrol potential of Bacillus subtilis. Appl. Microbiol. Biotechnol. 69, 29–38. doi: 10.1007/ s00253-005-1940-3
Ongena, M., and Jaques, P. (2008). Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol. 16, 115–125. doi: 10.1016/j.tim.2007.12. 009
Paik, S. H., Chakicherla, A., and Hansen, J. N. (1998). Identification and characterization of the structural and transporter genes for, and the chemical and biological properties of, sublancin 168, a novel lantibiotic produced by Bacillus subtilis 168. J. Biol. Chem. 273, 23134–23142. doi: 10.1074/jbc.273.36. 23134
Pan, L., Zhao, P. F., Ma, X. K., Shang, Q. H., Xu, Y. T., Long, S. F., et al. (2017). Probiotic supplementation protects weaned pigs against enterotoxigenic Escherichia coli K88 challenge and improves performance similar to antibiotics. J. Anim. Sci. 95, 2627–2639. doi: 10.2527/jas.2016.1243
Parkinson, T. J., Merrall, M., and Fenwick, S. G. (1999). A case of bovine mastitis caused by Bacillus cereus. N. Z. Vet. J. 47, 151–152.
Poulsen, A. S. R., de Jonge, N., Nielsen, J. L., Højberg, O., Lauridsen, C., Cutting, S. M., et al. (2018). Impact of Bacillus spp. spores and gentamicin on the gastrointestinal microbiota of suckling and newly weaned piglets. PLoS One 13:11. doi: 10.1371/journal.pone.0207382
Pu, J., Chen, D., Tian, G., He, J., Zheng, P., Mao, X., et al. (2018). Protective effects of benzoic acid, Bacillus coagulans, and oregano oil on intestinal injury caused by enterotoxigenic Escherichia coli in weaned piglets. Biomed. Res. Int. 2018:1829632. doi: 10.1155/2018/1829632
Rajput, I. R., Li, W. F., Li, Y. L., Jian, L., and Wang, M. Q. (2013). Application of probiotic (Bacillus subtilis) to enhance immunity, antioxidation, digestive enzymes activity and hematological profile of shaoxing duck. Pak. Vet. J. 33, 69–72.
Rishi, P., Mavi, S. K., Bharrhan, S., Shukla, G., and Tewari, R. (2009). Protective efficacy of probiotic alone or in conjunction with a prebiotic in Salmonella-induced liver damage. FEMS Microbiol. Ecol. 69, 222–230.
Roselli, M., Pieper, R., Rogel-Gaillard, C., de Vries, H., Bailey, M., Smidt, H., et al. (2017). Immunomodulating effects of probiotics for microbiota modulation, gut health and disease resistance in pigs. Anim. Feed Sci. Technol. 233, 104–119.
Savitri, L. P. (2021). “Probiotics for human health,” in Advances in Probiotics for Sustainable Food and Medicine. Microorganisms for Sustainability, Vol. 21, eds G. Goel and A. Kumar (Berlin: Springer), 181–212. doi: 10.1007/978-981-15- 6795-7_8
SCAN (2000). Opinion of the Scientific Committee on Animal Nutrition on the Safety of the Use of Bacillus Species in Animal Nutrition European Commission, Health and Consumer Protection Directorate-General. Scientific Committee on Animal Nutrition. Schieber, M., and Chandel, N. S. (2014). ROS function in redox signaling and oxidative stress. Curr. Biol. 24, R453–R462. doi: 10.1016/j.cub.2014.03.034
Schierack, P., Filter, M., Scharek, L., Toelke, C., Tara, D., Tedin, K., et al. (2009). Effect of Bacillus cereus var. toyoi on immune parameters of pregnant sows. Vet. Immunol. Immunopathol. 127, 26–37. doi: 10.1016/j.vetimm.2008.09.002
Shelburne, C. E., An, F. Y., Dholpe, V., Ramamoorthy, A., Lopatin, D. E., and Lantz, M. S. (2007). The spectrum of antimicrobial activity of the bacteriocin subtilosin A. J. Antimicrob. Chemother. 59, 297–300. doi: 10.1093/jac/dkl495
Siahmoshteh, F., Hamidi-Esfahani, Z., Spadaro, D., Shams-Ghahfarokhi, M., and Razzaghi-Abyaneh, M. (2018). Unraveling the mode of antifungal action of Bacillus subtilis and Bacillus amyloliquefaciens as potential biocontrol agents against aflatoxigenic Aspergillus parasiticus. Food Control 89, 300–307. doi: 10.1016/j.foodcont.2017.11.010
Sinol Sen, S., Ingale, S. L., Kim, J. S., Kim, K. H., Khong, C., Lohakare, J. D., et al. (2011). Effect of supplementation of Bacillus subtilis LS 1-2 grown on citrusjuice waste and corn-soybean meal substrate on growth performance, nutrient retention, caecal microbiology and small intestinal morphology of broilers. Asian-Australas. J. Anim. Sci. 24, 1120–1127.
Smitha, S., and Bhat, S. G. (2013). Thermostable Bacteriocin BL8 from Bacillus licheniformis isolated from marine sediment. J. Appl. Microbiol. 114, 688–694. doi: 10.1111/jam.12097
Song, B., Tang, D., Yan, S., Fan, H., Li, G., Shahid, M. S., et al. (2021). Effects of age on immune function in broiler chickens. J. Anim. Sci. Biotechnol. 12:42. doi: 10.1186/s40104-021-00559-1
Sutyak, K. E., Wirawan, R. E., Aroutcheva, A. A., and Chikindas, M. L. (2008). Isolation of the Bacillus subtilis antimicrobial peptide subtilosin from the dairy product-derived Bacillus amyloliquefaciens. J. Appl. Microbiol. 104, 1067–1074. doi: 10.1111/j.1365-2672.2007.03626.x
Tan, B. F., Lim, T., and Boontiam, W. (2020). Effect of dietary supplementation with essential oils and a Bacillus probiotic on growth performance, diarrhoea and blood metabolites in weaned pigs. Anim. Prod. Sci. 61, 64–71.
Tanaka, K., Takanaka, S., and Yoshida, K. I. (2014). A second-generation Bacillus cell factory for rare inositol production. Bioengineered 5, 331–334. doi: 10.4161/ bioe.29897
Tang, W., Qian, Y., Yu, B., Zhang, T., Gao, J., He, J., et al. (2019). Effects of Bacillus subtilis DSM32315 supplementation and dietary crude protein level on performance, gut barrier function and microbiota profile in weaned piglets. J. Anim. Sci. 97, 2125–2138. doi: 10.1093/jas/skz090
Taras, D., Vahjen, W., Macha, M., and Simon, O. (2005). Response of performance characteristics and fecal consistency to long-lasting dietary supplementation with the probiotic strain Bacillus cereus var. toyoi to sows and piglets. Arch. Anim. Nutr. 59, 405–417. doi: 10.1080/17450390500353168
Taras, D., Vahjen, W., and Simon, O. (2007). Probiotics in pigs — modulation of their intestinal distribution and of their impact on health and performance. Livest. Sci. 108, 229–231. doi: 10.1016/j.livsci.2007.01.075
Teo, A. Y. L., and Tan, H. M. (2006). Effect of Bacillus subtilis PB6 (CloSTAT) on broilers infected with a pathogenic strain of Escherichia coli. J. Appl. Poult. Res. 15, 229–235. doi: 10.1093/japr/15.2.229
Trela, J., Kieronczyk, B., Hautekiet, V., and Józefiak, D. (2020). Combination of ´ Bacillus licheniformis and Salinomycin: effect on the growth performance and git microbial populations of broiler chickens. Animals 10:889. doi: 10.3390/ ani10050889
Trevisi, P., Luise, D., Correa, F., and Bosi, P. (2021). Timely control of gastrointestinal eubiosis: a strategic pillar of pig health. Microorganisms 9:313. doi: 10.3390/microorganisms9020313
Tsukahara, T., Inatomi, T., Otomaru, K., Amatatsu, M., Romero-Pérez Gustavo, A., and Inoue, R. (2018). Probiotic supplementation improves reproductive performance of unvaccinated farmed sows infected with porcine epidemic diarrhea virus. Anim. Sci. J. 89, 1144–1151. doi: 10.1111/asj.13040
Vasilchenko, N., Kulikov, M., Stacenko, V., Pakhomov, V., Kulikova, N., and Gordeeva, N. (2022). “Regulation of nonribosomal peptide synthesis as a mechanism of antifungal activity of probiotics based on the bacteria genera bacillus and paenibacillus,” in XIV International Scientific Conference “INTERAGROMASH 2021". Lecture Notes in Networks and Systems, Vol. 246, eds A. Beskopylny and M. Shamtsyan (Cham: Springer), 111–121. doi: 10.1007/ 978-3-030-81619-3_12
Vilà, B., Esteve-Garcia, E., and Brufau, J. (2010). Probiotic micro-organisms: 100 years of innovation and efficacy; Modes of action. Worlds Poult. Sci. J. 66, 369–380. doi: 10.1017/s0043933910000474
Walsh, M. C., Rostagno, M. H., Gardiner, G. E., Sutton, A. L., Richert, B. T., and Radcliffe, J. S. (2012). Controlling Salmonella infection in weanling pigs through water delivery of direct-fed microbials or organic acids. Part I: effects on growth performance, microbial populations, and immune status. J. Anim. Sci. 90, 261–271. doi: 10.2527/jas.2010-3598
Wang, H., Kim, K. P., and Kim, I. H. (2019). Influence of Bacillus subtilis GCB-13- 001 on growth performance, nutrient digestibility, blood characteristics, faecal microbiota and faecal score in weanling pigs. J. Anim. Physiol. Anim. Nutr. (Berl). 103, 1919–1925. doi: 10.1111/jpn.13199
Wang, Q., Zhao, X., Chamu, J., and Shanmugam, K. T. (2011). Isolation, characterization and evolution of a new thermophilic Bacillus licheniformis for lactic acid production in mineral salts medium. Bioresour. Technol. 102, 8152–8158. doi: 10.1016/j.biortech.2011.06.003
Wang, X., Tian, Z., Azad, M. A. K., Zhang, W., Blachier, F., Wang, Z., et al. (2020). Dietary supplementation with Bacillus mixture modifies the intestinal ecosystem of weaned piglets in an overall beneficial way. J. Appl. Microbiol. 130, 233–246. doi: 10.1111/jam.14782
Wang, X., Tsai, T., Wei, X., Zuo, B., Davis, E., Rehberger, T., et al. (2021). Effect of lactylate and Bacillus subtilis on growth performance, peripheral blood cell profile, and gut microbiota of nursery pigs. Microorganisms 9:803. doi: 10.3390/ microorganisms9040803
Whelan, R. A., Doranalli, K., Rinttilä, T., Vienola, K., Jurgens, G., and Apajalahti, J. (2019). The impact of Bacillus subtilis DSM 32315 on the pathology, performance, and intestinal microbiome of broiler chickens in a necrotic enteritis challenge. Poult. Sci. 98, 3450–3463. doi: 10.3382/ps/pey500
Wu, L., Liao, P., He, L., Ren, W., Yin, J., Duan, J., et al. (2015). Growth performance, serum biochemical profile, jejunal morphology, and the expression of nutrients transporter genes in deoxynivalenol (DON)- challenged growing pigs. BMC Vet. Res. 11:144. doi: 10.1186/s12917-015-0449-y
Wu, S. B., Stanley, D., Rodgers, N., Swick, R. A., and Moore, R. J. (2014). Two necrotic enteritis predisposing factors, dietary fish- meal and Eimeria infection, induce large changes in the caecal microbiota of broiler chickens. Vet. Microbiol. 169, 188–197. doi: 10.1016/j.vetmic.2014.01.007
Xu, L., Fan, Q., Zhuang, Y., Wang, Q., Gao, Y., and Wang, C. (2017). Bacillus coagulans enhance the immune function of the intestinal mucosa of yellow broilers. Rev. Bras. Cienc. Avic. 19, 115–122. doi: 10.1590/1806-9061-2015-0180
Xu, J., Zhong, F., Zhang, Y., Zhang, J., Huo, S., Lin, H., et al. (2017). Construction of Bacillus subtilis strain engineered for expression of porcine β-defensin2/cecropin P1 fusion antimicrobial peptides and its growth-promoting effect and antimicrobial activity. Asian-Australas. J. Anim. Sci. 30, 576–584. doi: 10. 5713/ajas.16.0207
Xu, X., Huang, Q., Mao, Y., Cui, Z., Li, Y., Huang, Y., et al. (2012). Immunomodulatory effects of Bacillus subtilis (natto) B4 spores on murine macrophages. Microbiol. Immunol. 56, 817–824. doi: 10.1111/j.1348-0421.2012. 00508.x
Zhang, B., Zhang, H., Yu, Y., Zhang, R., Wu, Y., Yue, M., et al. (2021). Effects of Bacillus coagulans on growth performance, antioxidant capacity, immunity function, and gut health in broilers. Poult. Sci. 100:101168. doi: 10.1016/j.psj. 2021.101168
Zhang, Q., Li, J., Cao, M., Li, Y., Zhuo, Y., Fang, Z., et al. (2020). Dietary supplementation of Bacillus subtilis PB6 improves sow reproductive performance and reduces piglet birth intervals. Anim. Nutr. 6, 278–287. doi: 10.1016/j.aninu.2020.04.002
Zhao, Y., Zeng, D., Wang, H., Qing, X., Sun, N., Xin, J., et al. (2020). Dietary probiotic Bacillus licheniformis h2 enhanced growth performance, morphology of small intestine and liver, and antioxidant capacity of broiler chickens against Clostridium perfringens–induced subclinical necrotic enteritis. Probiotics Antimicrob. Proteins 12, 883–895. doi: 10.1007/s12602-019-09 597-8
Zheng, G., and Slavik, M. F. (1999). Isolation, partial purification and characterization of a bacteriocin produced by a newly isolated Bacillus subtilis strain. Lett. Appl. Microbiol. 28, 363–367. doi: 10.1046/j.1365- 2672.1999.00545.x
Zhou, D., Zhu, Y. H., Zhang, W., Wang, M. L., Fan, W. Y., Song, D., et al. (2015). Oral administration of a select mixture of Bacillus probiotics generates Tr1 cells in weaned F4ab/acR- pigs challenged with an F4+ ETEC/VTEC/EPEC strain. Vet. Res. 46:95. doi: 10.1186/s13567-015- 0223-y
Zhou, J., Ao, X., Lei, Y., Ji, C., and Ma, Q. (2020). Bacillus subtilis ANSB01G culture alleviates oxidative stress and cell apoptosis induced by dietary zearalenone in first-parity gestation sows. Anim. Nutr. 6, 372–378. doi: 10.1016/j.aninu.2020. 03.011
Zong, X., Wang, T. H., Lu, Z. Q., Song, D. G., Zhao, J., and Wang, Y. Z. (2019). Effects of Clostridium butyricum or in combination with Bacillus licheniformis on the growth performance, blood indexes, and intestinal barrier function of weanling piglets. Livest. Sci. 220, 137–142. doi: 10.1016/j.livsci.2018.12.024











.jpg&w=3840&q=75)