Feeding strategy for alleviating the adverse effects of heat stress
Thermal Comfort, Growth Performance and Welfare of Olive Pulp Fed Broilers during Hot Season
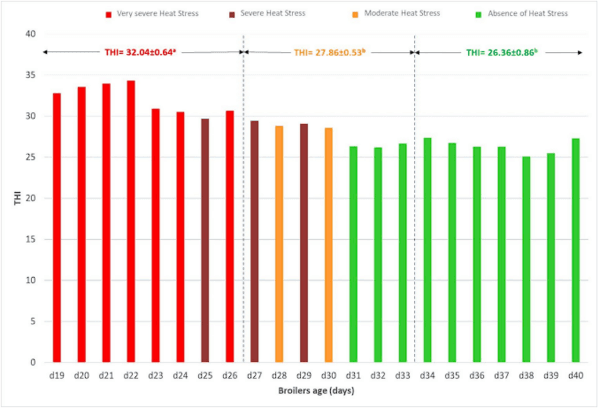
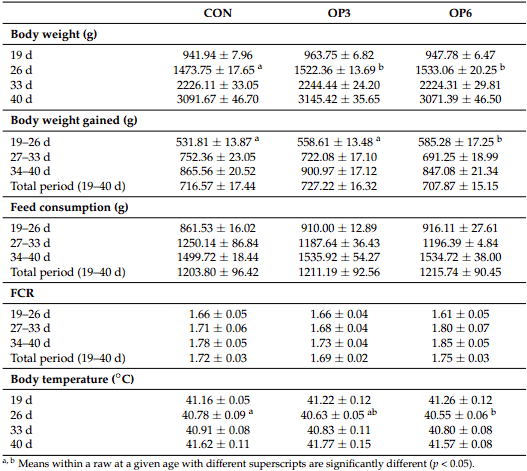
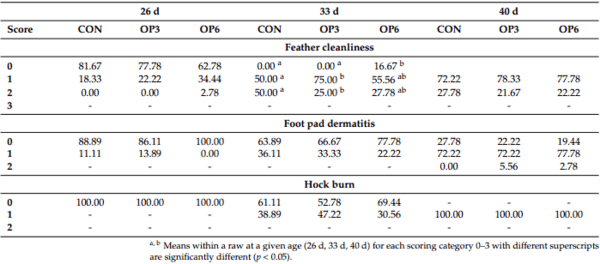
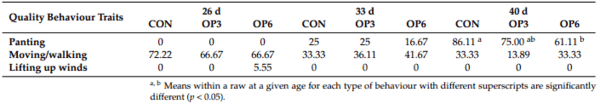
1. Marangoni, F.; Corsello, G.; Cricelli, C.; Ferrara, N.; Ghiselli, A.; Lucchin, L.; Poli, A. Role of poultry meat in a balanced diet aimed at maintaining health and wellbeing: An Italian consensus document. Food Nutr. Res. 2015, 59, 27606. [CrossRef]
2. American Egg Board. Egg Protein Comparision and Costs. Available online: https://www.aeb.org/farmersand-marketers/ protein-comparison (accessed on 12 January 2023).
3. Searchinger, T.; Waite, R.; Hanson, C.; Ranganathan, J. Creating a Sustainable Food Future: A Menu of Solutions to Feed Nearly 10 billion People by 2050; Final report; World Resources Institute: Washington, DC, USA, 2019.
4. PRB. Population Reference Bureau: World Population Data Sheet 2020; Library of Congress: Washington, DC, USA, 2020.
5. Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; ESA Working paper No. 12-03; FAO: Rome, Italy, 2012.
6. Sz˝oll˝osi, L. Current State and Future Prospects of the Egg Sector—An International Outlook. Agric. Conspec. Sci. 2021, 86, 95–105.
7. European Parliament. Directorate General for Parliamentary Research Services. In The EU Poultry Meat and Egg Sector: Main Features, Challenges and Prospects: In Depth Analysis; Publications Office: Luxembourg, 2019.
8. Lara, L.; Rostagno, M. Impact of Heat Stress on Poultry Production. Animals 2013, 3, 356–369. [CrossRef] [PubMed]
9. Teyssier, J.-R.; Brugaletta, G.; Sirri, F.; Dridi, S.; Rochell, S.J. A review of heat stress in chickens. Part II: Insights into protein and energy utilization and feeding. Front. Physiol. 2022, 13, 943612. [CrossRef] [PubMed]
10. Naga Raja Kumari, K.; Narendra Nath, D. Ameliorative measures to counter heat stress in poultry. World’s Poult. Sci. J. 2018, 74, 117–130. [CrossRef]
11. Abdel-Moneim, A.-M.E.; Shehata, A.M.; Khidr, R.E.; Paswan, V.K.; Ibrahim, N.S.; El-Ghoul, A.A.; Aldhumri, S.A.; Gabr, S.A.; Mesalam, N.M.; Elbaz, A.M.; et al. Nutritional manipulation to combat heat stress in poultry—A comprehensive review. J. Therm. Biol. 2021, 98, 102915. [CrossRef]
12. St-Pierre, N.R.; Cobanov, B.; Schnitkey, G. Economic Losses from Heat Stress by US Livestock Industries. J. Dairy Sci. 2003, 86, E52–E77. [CrossRef]
13. Masson-Delmotte, V.; Zhai, P.; Pirani, A. Climate Change 2021: The Physical Science Basis: Summary for Policymakers: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2021; ISBN 978-92-9169-158-6.
14. Wasti, S.; Sah, N.; Mishra, B. Impact of Heat Stress on Poultry Health and Performances, and Potential Mitigation Strategies. Animals 2020, 10, 1266. [CrossRef]
15. Hu, R.; He, Y.; Arowolo, M.; Wu, S.; He, J. Polyphenols as Potential Attenuators of Heat Stress in Poultry Production. Antioxidants 2019, 8, 67. [CrossRef]
16. Goel, A. Heat stress management in poultry. J. Anim. Physiol. Anim. Nutr. 2021, 105, 1136–1145. [CrossRef]
17. Visioli, F.; Poli, A.; Gall, C. Antioxidant and other biological activities of phenols from olives and olive oil. Med. Res. Rev. 2002, 22, 65–75. [CrossRef]
18. Omar, S.H. Oleuropein in Olive and its Pharmacological Effects. Sci. Pharm. 2010, 78, 133–154. [CrossRef]
19. Barbaro, B.; Toietta, G.; Maggio, R.; Arciello, M.; Tarocchi, M.; Galli, A.; Balsano, C. Effects of the Olive-Derived Polyphenol Oleuropein on Human Health. IJMS 2014, 15, 18508–18524. [CrossRef]
20. Bulotta, S.; Celano, M.; Lepore, S.M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: Focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 219. [CrossRef] [PubMed]
21. Casamenti, F.; Grossi, C.; Rigacci, S.; Pantano, D.; Luccarini, I.; Stefani, M. Oleuropein Aglycone: A Possible Drug against Degenerative Conditions. In Vivo Evidence of its Effectiveness against Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 45, 679–688. [CrossRef] [PubMed]
22. Khwaldia, K.; Attour, N.; Matthes, J.; Beck, L.; Schmid, M. Olive byproducts and their bioactive compounds as a valuable source for food packaging applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1218–1253. [CrossRef] [PubMed]
23. Zangeneh, S.; Torki, M. Effects of B-Mannanase Supplementing of Olive Pulp-Included Diet on Performance of Laying Hens, Egg Quality Characteristics, Humoral and Cellular Immune Response and Blood Parameters. Glob. Vet. 2011, 7, 391–398.
24. Salomone, R.; Ioppolo, G. Environmental impacts of olive oil production: A Life Cycle Assessment case study in the province of Messina (Sicily). J. Clean. Prod. 2012, 28, 88–100. [CrossRef]
25. Lillehoj, H.; Liu, Y.; Calsamiglia, S.; Fernandez-Miyakawa, M.E.; Chi, F.; Cravens, R.L.; Oh, S.; Gay, C.G. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 2018, 49, 76. [CrossRef]
26. Upadhaya, S.D.; Kim, I.H. Efficacy of Phytogenic Feed Additive on Performance, Production and Health Status of Monogastric Animals—A Review. Ann. Anim. Sci. 2017, 17, 929–948. [CrossRef]
27. European Commission. Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions. The European Green Deal. 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2019%3A640%3AFIN (accessed on 1 February 2022).
28. Ibrahim, N.S.; Sabic, E.M.; Abu-Taleb, A.M. Effect of inclusion irradiated olive pulp in laying quail diets on biological performance. J. Radiat. Res. Appl. Sci. 2018, 11, 340–346. [CrossRef]
29. Sayehban, P.; Seidavi, A.; Dadashbeiki, M.; Ghorbani, A.; Araújo, W.A.G.; Albino, L.F.T. Effects of Different Levels of Two Types of Olive Pulp with or without Exogenous Enzyme Supplementation on Broiler Performance and Economic Parameters. Rev. Bras. Cienc. Avic. 2016, 18, 489–500. [CrossRef]
30. Benavente-García, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Rio, J.A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [CrossRef]
31. Rafehi, H.; Ververis, K.; Karagiannis, T.C. Mechanisms of Action of Phenolic Compounds in Olive. J. Diet. Suppl. 2012, 9, 96–109. [CrossRef]
32. Ranalli, A.; Pollastri, L.; Contento, S.; Di Loreto, G.; Iannucci, E.; Lucera, L.; Russi, F. Acylglycerol and Fatty Acid Components of Pulp, Seed, and Whole Olive Fruit Oils. Their Use to Characterize Fruit Variety by Chemometrics. J. Agric. Food Chem. 2002, 50, 3775–3779. [CrossRef]
33. Tufarelli, V.; Introna, M.; Cazzato, E.; Mazzei, D.; Laudadio, V. Suitability of partly destoned exhausted olive cake as by-product feed ingredient for lamb production. J. Anim. Sci. 2013, 91, 872–877. [CrossRef]
34. Rabayaa, E.; Omar, J.M.A.; Othman, R.A. Utilization of Olive Pulp in Broiler Rations. An-Najah Univ. J. Res. 2001, 15, 133–144. [CrossRef]
35. Mohamed, M.E.; Ali, A.M.; Shetaewi, M.M.; Said, K.I.; Abdel-Ghaffar, M.A. Utilization of Olive Pomace Meal and Orange By-Product Meal In Broiler Rations. Sinai J. Appl. Sci. 2013, 2, 113–128. [CrossRef]
36. Al-Harthi, M.A. The efficacy of using olive cake as a by-product in broiler feeding with or without yeast. Ital. J. Anim. Sci. 2016, 15, 512–520. [CrossRef]
37. Al-Harthi, M. The Effect of Olive Cake, with or Without Enzymes Supplementation, on Growth Performance, Carcass Characteristics, Lymphoid Organs and Lipid Metabolism of Broiler Chickens. Rev. Bras. Cienc. Avic. 2017, 19, 83–90. [CrossRef]
38. Sateri, S.; Seidavi, A.; Bouyeh, M.; Neumann, P.; Kutzler, M.; Laudadio, V.; Loperfido, F.; Tufarelli, V. Effect of olive meal and supplemental enzymes on performance traits, blood biochemistry, humoral immunity response and caecal microbiota of broilers. SA J. An. Sci. 2017, 47, 804. [CrossRef]
39. Papadomichelakis, G.; Pappas, A.C.; Tsiplakou, E.; Symeon, G.K.; Sotirakoglou, K.; Mpekelis, V.; Fegeros, K.; Zervas, G. Effects of dietary dried olive pulp inclusion on growth performance and meat quality of broiler chickens. Livest. Sci. 2019, 221, 115–122. [CrossRef]
40. Pappas, A.C.; Tsiplakou, E.; Papadomichelakis, G.; Mitsiopoulou, C.; Sotirakoglou, K.; Mpekelis, V.; Haroutounian, S.A.; Fegeros, K.; Zervas, G. Effects of olive pulp addition to broiler diets on performance, selected biochemical parameters and antioxidant enzymes. J. Hell. Vet. Med. Soc. 2019, 70, 1687–1696. [CrossRef]
41. ELbaz, A.M.; Thabet, H.; Gad, G. Productive and Physiological Performance of Broilers Fed Diets Containing Different Levels of Olive Pulp. J. Anim. Poult. Prod. 2020, 11, 435–439. [CrossRef]
42. Saleh, A.A.; Paray, B.A.; Dawood, M.A.O. Olive Cake Meal and Bacillus licheniformis Impacted the Growth Performance, Muscle Fatty Acid Content, and Health Status of Broiler Chickens. Animals 2020, 10, 695. [CrossRef] [PubMed]
43. Fotou, E.; Moulasioti, V.; Angelis, I.; Tsiouris, V.; Patsias, A.; Tellis, C.; Moussis, V.; Boti, M.; Tsoukatos, D. Influence of dietary olive paste flour on the performance and oxidative stress in chickens raised in field conditions. J. Hell. Vet. Med. Soc. 2021, 72, 3239–3248. [CrossRef]
44. Dedousi, A.; Kotzamanidis, C.; Kritsa, M.-Z.; Tsoureki, A.; Andreadelli, A.; Patsios, S.I.; Sossidou, E. Growth Performance, Gut Health, Welfare and Qualitative Behavior Characteristics of Broilers Fed Diets Supplemented with Dried Common (Olea europaea) Olive Pulp. Sustainability 2022, 15, 501. [CrossRef]
45. Mujahid, A.; Akiba, Y.; Toyomizu, M. Olive oil-supplemented diet alleviates acute heat stress-induced mitochondrial ROS production in chicken skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R690–R698. [CrossRef]
46. Rafei-Tari, A.; Sadeghi, A.A.; Mousavi, S.N. Inclusion of vegetable oils in diets of broiler chicken raised in hot weather and effects on antioxidant capacity, lipid components in the blood and immune responses. Acta Sci. Anim. Sci. 2021, 43, e50587. [CrossRef]
47. Tari, A.R.; Sadeghi, A.A.; Mousavi, S.N. Dietary vegetable oils inclusion on the performance, hormonal levels and hsp 70 gene expression in broilers under heat stress. Acta Sci. Anim. Sci. 2019, 42, e45517. [CrossRef]
48. Oke, O.E.; Emeshili, U.K.; Iyasere, O.S.; Abioja, M.O.; Daramola, J.O.; Ladokun, A.O.; Abiona, J.A.; Williams, T.J.; Rahman, S.A.; Rotimi, S.O.; et al. Physiological responses and performance of broiler chickens offered olive leaf extract under a hot humid tropical climate. J. Appl. Poult. Res. 2017, 26, 376–382. [CrossRef]
49. Agah, M.J.; Mirakzehi, M.T.; Saleh, H. Effects of olive leaf extract (Olea Europea L.) on growth performance, blood metabolites and antioxidant activities in broiler chickens under heat stress. J. Anim. Plant Sci. 2020, 29, 9.
50. EUR-Lex. COUNCIL DIRECTIVE 2007/43/EC of 28 June 2007 Laying down Minimum Rules for the Protection of Chickens Kept for Meat Production. 2007. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX: 32007L0043&from=EN (accessed on 22 December 2022).
51. Cobb-Broiler Management Guide. Available online: https://www.cobb-vantress.com/assets/5c7576a214/Broiler-guide-R1.pdf (accessed on 12 April 2023).
52. Marai, I.F.M.; Ayyat, M.S.; Abd El-Monem, U.M. Growth Performance and Reproductive Traits at First Parity of New Zealand White Female Rabbits as Affected by Heat Stress and Its Alleviation under Egyptian Conditions. Trop. Anim. Health Prod. 2001, 33, 451–462. [CrossRef]
53. Habeeb, A.A.; Gad, A.E.; Atta, M.A. Temperature-Humidity Indices as Indicators to Heat Stress of Climatic Conditions with Relation to Production and Reproduction of Farm Animals. Int. J. Biotechnol. Recent. Adv. 2018, 1, 35–50. [CrossRef]
54. McCafferty, D.J.; Gallon, S.; Nord, A. Challenges of measuring body temperatures of free-ranging birds and mammals. Anim. Biotelemetry 2015, 3, 33. [CrossRef]
55. Welfare Quality®. Welfare Quality®Assessment Protocol for Poultry (Broilers, Laying Hens); Welfare Quality®Consortium: Lelystad, The Netherlands, 2009.
55. Welfare Quality®. Welfare Quality®Assessment Protocol for Poultry (Broilers, Laying Hens); Welfare Quality®Consortium: Lelystad, The Netherlands, 2009.
57. Attia, Y.A.; Al-Harthi, M.A.; Hassan, S.S. Responses of broiler chicken to different oil levels within constant energy levels from 20 to 40 days of age under hot weather conditions. Ital. J. Anim. Sci. 2021, 20, 664–676. [CrossRef]
58. Kpomasse, C.C.; Oke, O.E.; Houndonougbo, F.M.; Tona, K. Broiler production challenges in the tropics: A review. Vet. Med. Sci. 2021, 7, 831–842. [CrossRef]
59. Shakeri, M.; Le, H.H. Deleterious Effects of Heat Stress on Poultry Production: Unveiling the Benefits of Betaine and Polyphenols. Poultry 2022, 1, 147–156. [CrossRef]
60. Abdel-Moneim, A.E.; Shehata, A.M.; Alzahrani, S.O.; Shafi, M.E.; Mesalam, N.M.; Taha, A.E.; Swelum, A.A.; Arif, M.; Fayyaz, M.; Abd El-Hack, M.E. The role of polyphenols in poultry nutrition. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1851–1866. [CrossRef] [PubMed]
61. Brenes, A.; Viveros, A.; Chamorro, S.; Arija, I. Use of polyphenol-rich grape by-products in monogastric nutrition. A review. Anim. Feed. Sci. Technol. 2016, 211, 1–17. [CrossRef]
62. Shakeri, M.; Cottrell, J.J.; Wilkinson, S.; Le, H.H.; Suleria, H.A.R.; Warner, R.D.; Dunshea, F.R. A Dietary Sugarcane-Derived Polyphenol Mix Reduces the Negative Effects of Cyclic Heat Exposure on Growth Performance, Blood Gas Status, and Meat Quality in Broiler Chickens. Animals 2020, 10, 1158. [CrossRef]
63. Paszkiewicz, M.; Budzy ´nska, A.; Rózalska, B.; Sadowska, B. The immunomodulatory role of plant polyphenols. ˙ Postep. Hig. Med. Dosw. 2012, 66, 637–646. [CrossRef]
64. Rehman, K.; Haider, K.; Jabeen, K.; Akash, M.S.H. Current perspectives of oleic acid: Regulation of molecular pathways in mitochondrial and endothelial functioning against insulin resistance and diabetes. Rev. Endocr. Metab. Disord. 2020, 21, 631–643. [CrossRef]
65. Branciari, R.; Galarini, R.; Giusepponi, D.; Trabalza-Marinucci, M.; Forte, C.; Roila, R.; Miraglia, D.; Servili, M.; Acuti, G.; Valiani, A. Oxidative Status and Presence of Bioactive Compounds in Meat from Chickens Fed Polyphenols Extracted from Olive Oil Industry Waste. Sustainability 2017, 9, 1566. [CrossRef]
66. Safwat, G.M.; Kandiel, M.A.; Abozaid, O.A.R.; Arafa, M.M.; Mohamed, S.O. Biochemical Effect of Olive Cake as Feed Additive on Antioxidants and Molecular Expression of FAS, ANS, ACC in Laying Hens. Adv. Anim. Vet. Sci. 2022, 10, 731–738. [CrossRef]
67. Zubair, A.K.; Leeson, S. Compensatory growth in the broiler chicken: A review. World’s Poult. Sci. J. 1996, 52, 189–201. [CrossRef]
68. Saleh, A.; Alzawqari, M. Effects of Replacing Yellow Corn with Olive Cake Meal on Growth Performance, Plasma Lipid Profile, and Muscle Fatty Acid Content in Broilers. Animals 2021, 11, 2240. [CrossRef] [PubMed]
69. Joven, M.; Pintos, E.; Latorre, M.A.; Suárez-Belloch, J.; Guada, J.A.; Fondevila, M. Effect of replacing barley by increasing levels of olive cake in the diet of finishing pigs: Growth performances, digestibility, carcass, meat and fat quality. Anim. Feed. Sci. Technol. 2014, 197, 185–193. [CrossRef]
70. Saraiva, S.; Saraiva, C.; Stilwell, G. Feather conditions and clinical scores as indicators of broilers welfare at the slaughterhouse. Res. Vet. Sci. 2016, 107, 75–79. [CrossRef] [PubMed]
71. Arnould, C.; Butterworth, A.; Knierim, U. Standardisation of clinical scoring in poultry. In Assessment of Animal Welfare Measures for Layers and Broilers; Forkman, B., Keeling, L., Eds.; School of City and Regional Planning, Cardiff University: Cardiff, UK, 2009; pp. 7–30. ISBN 978-1-902647-79-1.
72. Berg, C.C. Foot-pad dermatitis in broilers and turkeys. In Prevalence, Risk Factors and Prevention; Doctoral Thesis, Swedish University of Agricultural Sciences: Uppsala, Sweden, 1998.
73. Weaver, W.D.; Meijerhof, R. The Effect of Different Levels of Relative Humidity and Air Movement on Litter Conditions, Ammonia Levels, Growth, and Carcass Quality for Broiler Chickens. Poult. Sci. 1991, 70, 746–755. [CrossRef]
74. Bruce, D.W.; McIlroy, S.G.; Goodall, E.A. Epidemiology of a contact dermatitis of broilers. Avian Pathol. 1990, 19, 523–537. [CrossRef]
75. Brugaletta, G.; Teyssier, J.-R.; Rochell, S.J.; Dridi, S.; Sirri, F. A review of heat stress in chickens. Part I: Insights into physiology and gut health. Front. Physiol. 2022, 13, 934381. [CrossRef] [PubMed]
76. Nawaz, A.H.; Amoah, K.; Leng, Q.Y.; Zheng, J.H.; Zhang, W.L.; Zhang, L. Poultry Response to Heat Stress: Its Physiological, Metabolic, and Genetic Implications on Meat Production and Quality Including Strategies to Improve Broiler Production in a Warming World. Front. Vet. Sci. 2021, 8, 699081. [CrossRef] [PubMed]
77. Manning, L.; Chadd, S.A.; Baines, R.N. Water consumption in broiler chicken: A welfare indicator. World’s Poult. Sci. J. 2007, 63, 63–71. [CrossRef]
78. Grimes, J.L.; Smith, J.; Williams, C.M. Some alternative litter materials used for growing broilers and turkeys. World’s Poult. Sci. J. 2002, 58, 515–526. [CrossRef]
79. Sanotra, G.S.; Berg, C.; Lund, J.D. A Comparison Between Leg Problems in Danish and Swedish Broiler Production. Anim. Welf. 2003, 12, 677–683. [CrossRef]
80. Bilgili, S.F.; Alley, M.A.; Hess, J.B.; Nagaraj, M. Influence of Age and Sex on Footpad Quality and Yield in Broiler Chickens Reared on Low and High Density Diets. J. Appl. Poult. Res. 2006, 15, 433–441. [CrossRef]
81. Zampiga, M.; Meluzzi, A.; Pignata, S.; Sirri, F. Occurrence of Breast Meat Abnormalities and Foot Pad Dermatitis in Light-Size Broiler Chicken Hybrids. Animals 2019, 9, 706. [CrossRef]
82. Kestin, S.; Su, G.; Sorensen, P. Different commercial broiler crosses have different susceptibilities to leg weakness. Poult. Sci. 1999, 78, 1085–1090. [CrossRef]
83. Yahav, S.; Collin, A.; Shinder, D.; Picard, M. Thermal manipulations during broiler chick embryogenesis: Effects of timing and temperature. Poult. Sci. 2004, 83, 1959–1963. [CrossRef]
84. Borges, S.A.; Da Silva, A.V.F.; Maiorka, A. Acid-base balance in broilers. World’s Poult. Sci. J. 2007, 63, 73–81. [CrossRef]
85. McLean, J.A.; Savory, C.J.; Sparks, N.H.C. Welfare of Male and Female Broiler Chickens in Relation to Stocking Density, as Indicated by Performance, Health and Behaviour. Anim. Welf. 2002, 11, 55–73. [CrossRef]
86. Akter, S.; Liu, Y.; Cheng, B.; Classen, J.; Oviedo, E.; Harris, D.; Wang-Li, L. Impacts of Air Velocity Treatments under Summer Conditions: Part II—Heavy Broiler’s Behavioral Response. Animals 2022, 12, 1050. [CrossRef]
87. Mahmoud, U.T.; Abdel-Rahman, M.A.M.; Darwish, M.H.A.; Applegate, T.J.; Cheng, H. Behavioral changes and feathering score in heat stressed broiler chickens fed diets containing different levels of propolis. Appl. Anim. Behav. Sci. 2015, 166, 98–105. [CrossRef]
88. Lucini Mas, A.; Bonansea, R.I.; Fernandez, M.E.; Kembro, J.M.; Labaque, M.C.; Wunderlin, D.A.; Baroni, M.V. Dietary supplementation with chia polyphenols alleviates oxidative stress and improves egg nutritional quality in Japanese quails under heat stress. J. Therm. Biol. 2023, 111, 103421. [CrossRef] [PubMed]
89. Egbuniwe, I.C.; Ayo, J.O.; Ocheja, O.B. Betaine and ascorbic acid modulate indoor behavior and some performance indicators of broiler chickens in response to hot-dry season. J. Therm. Biol. 2018, 76, 38–44. [CrossRef]
90. Zmrhal, V.; Lichovníková, M.; Hampel, D. The Effect of Phytogenic Additive on Behavior During Mild-Moderate Heat Stress in Broilers. Acta Univ. Agric. Silvic. Mendel. Brun. 2018, 66, 939–944. [CrossRef]









.jpg&w=3840&q=75)