Effects of Dietary Betaine on the Secretion of Insulin-like Growth Factor-I and Insulin-like Growth Factor Binding Protein-1 and -3 in Laying Hens
Published: October 19, 2010
By: H. S. Choe, H. L. Li, (Yanbian University) J. H. Park, C. W. Kang and K. S. Ryu (Chonbuck National University)
ABSTRACT: The principal objective of this experiment was to determine the effects of dietary betaine on IGF-I, IGFBP-3 and IGFBP-1 secretion and IGF-I mRNA gene expression in the serum and liver of laying hens. A total of 72 ISA-Brown laying hens were fed with four different levels of betaine (0, 300, 600, 1,200 ppm) based on a corn-soybean meal diet containing 2,800 kcal/kg of metabolizable energy (ME) and 16% crude protein (CP) for four weeks. The results indicated significantly higher serum and liver IGF-I concentrations in the laying hens fed with 600 and 1,200 ppm betaine (p<0.05) compared to controls. IGF-I gene expression in liver showed a statistically correlated increase in 600 and 1,200 ppm betaine-fed groups as compared to the controls (p<0.05). Serum IGFBP-3 concentrations were elevated significantly in the groups fed 600 ppm of betaine. However, the secretion of IGFBP-1 in the liver of laying hens fed on 600 and 1,200 ppm of betaine was significantly lower than in the controls (p<0.05). The results of this experiment showed that dietary betaine supplementation plays a pivotal role in changes of the IGFs system in laying hens.
INTRODUCTION
Betaine has been well-established as a labile methyl donor and as an osmoprotectant, which is synthesized via the oxidation of betaine aldehyde formed by the irreversible oxidation of choline in the mitochondria of the liver and renal tissues (Kidd et al., 1997, Zulkifli et al., 2004; Honarbakhsh et al., 2007). Betaine is involved in the methionine cycle, a physiologically relevant transmethylation reaction, in which a methyl group is donated to homocysteine (Nocianitri et al., 2002). Thus, betaine has been considered to stimulate methionine synthesis or replace some crucial functions of methionine, and may also activate S-adenosyl methionine (S-AM) for effective hepatocyte regeneration (Kettunen et al., 2001). The various effects of betaine on animal metabolism have been elucidated, including a direct effect on hormone secretion in the control of osmotic pressure in the rat, as well as in the prevention of liver damage via the activation of reactive oxygen species (ROS), as reported by Barak et al. (1994). Betaine is added to the feed of laying hens at the level of 600 ppm to significantly improve laying performance compared to controls. So, it consequently contributes to enhanced productivity which could have an important economic value (Park et al., 2006). On the other hand, insulin-like growth factor-I (IGF-I) performs a crucial function in vertebrate growth. It is a polypeptide consisting of 70 amino acids with a structure similar to that of pro-insulin, and it also affects several important metabolic processes inherent to the growth and differentiation of cells (Froesch et al., 1985; Olivecrona et al., 1999). IGF-I is present along with its binding proteins (IGFBPs) in blood and with receptors on the surfaces of cells. These binding proteins lengthen the half-life of IGFs, and deliver it to target cells. Among these IGFBPs, IGFBP-3 is known to perform the function of cell proliferation, differentiation, immunity, and cellular remodeling of organs in vivo (Jones and Clemmons, 1995), and IGFBP-1 performs a function in relation to stress and immunity (Lee et al., 1993; Jones and Clemmons, 1995).
Although a variety of mechanisms affecting the secretion of IGF-I and IGFBPs have been widely reported in vertebrate studies, factors affecting the hormonal secretion in poultry have not been reported yet. Therefore, the current study was conducted to investigate the role of betaine for its effect on the secretion characteristics and secretion pattern of IGF-I and IGFBPs in laying hens.
INTRODUCTION
Betaine has been well-established as a labile methyl donor and as an osmoprotectant, which is synthesized via the oxidation of betaine aldehyde formed by the irreversible oxidation of choline in the mitochondria of the liver and renal tissues (Kidd et al., 1997, Zulkifli et al., 2004; Honarbakhsh et al., 2007). Betaine is involved in the methionine cycle, a physiologically relevant transmethylation reaction, in which a methyl group is donated to homocysteine (Nocianitri et al., 2002). Thus, betaine has been considered to stimulate methionine synthesis or replace some crucial functions of methionine, and may also activate S-adenosyl methionine (S-AM) for effective hepatocyte regeneration (Kettunen et al., 2001). The various effects of betaine on animal metabolism have been elucidated, including a direct effect on hormone secretion in the control of osmotic pressure in the rat, as well as in the prevention of liver damage via the activation of reactive oxygen species (ROS), as reported by Barak et al. (1994). Betaine is added to the feed of laying hens at the level of 600 ppm to significantly improve laying performance compared to controls. So, it consequently contributes to enhanced productivity which could have an important economic value (Park et al., 2006). On the other hand, insulin-like growth factor-I (IGF-I) performs a crucial function in vertebrate growth. It is a polypeptide consisting of 70 amino acids with a structure similar to that of pro-insulin, and it also affects several important metabolic processes inherent to the growth and differentiation of cells (Froesch et al., 1985; Olivecrona et al., 1999). IGF-I is present along with its binding proteins (IGFBPs) in blood and with receptors on the surfaces of cells. These binding proteins lengthen the half-life of IGFs, and deliver it to target cells. Among these IGFBPs, IGFBP-3 is known to perform the function of cell proliferation, differentiation, immunity, and cellular remodeling of organs in vivo (Jones and Clemmons, 1995), and IGFBP-1 performs a function in relation to stress and immunity (Lee et al., 1993; Jones and Clemmons, 1995).
Although a variety of mechanisms affecting the secretion of IGF-I and IGFBPs have been widely reported in vertebrate studies, factors affecting the hormonal secretion in poultry have not been reported yet. Therefore, the current study was conducted to investigate the role of betaine for its effect on the secretion characteristics and secretion pattern of IGF-I and IGFBPs in laying hens.
MATERIALS AND METHODS
Experimental design
A total of 72, 70 week-old ISA-Brown commercial laying hens were placed individually in cages and provided with 4 different concentrations of dietary betaine (0, 300, 600, 1,200 ppm) for 4 weeks. The basal diet was predominantly corn-soybean meal and contained 2,800 kcal/kg of ME and 16% CP (Table 1).
The laying hens were fed ad libitum during the entire test period. Daylight was provided for 18 h. At the end of the experiment, blood was harvested from all hens which were sacrificed via cervical dislocation, after which the livers were removed. The samples were maintained at 70ºC prior to analysis. The concentrations of IGF-I, IGF-mRNA, IGFBP-3 and IGFBP-1 in the liver and blood were measured via the analytical methods described below.
A total of 72, 70 week-old ISA-Brown commercial laying hens were placed individually in cages and provided with 4 different concentrations of dietary betaine (0, 300, 600, 1,200 ppm) for 4 weeks. The basal diet was predominantly corn-soybean meal and contained 2,800 kcal/kg of ME and 16% CP (Table 1).
The laying hens were fed ad libitum during the entire test period. Daylight was provided for 18 h. At the end of the experiment, blood was harvested from all hens which were sacrificed via cervical dislocation, after which the livers were removed. The samples were maintained at 70ºC prior to analysis. The concentrations of IGF-I, IGF-mRNA, IGFBP-3 and IGFBP-1 in the liver and blood were measured via the analytical methods described below.
IGF-I radioimmunoassay
Recombinant chicken IGF-I was iodinated to a specific radioactivity of 150-300 Ci/g with the isotope I125, using a modified version of the chloramin-T (Kodak, Grand Island, NY, USA) technique (Lee and Henricks, 1990). IGF-I immunoreactivity was determined via the techniques developed by Lee et al. (1991). All assays were conducted in duplicate. The intra- and inter-assay coefficients of variation for IGFs were 8% and 10%, respectively.
Recombinant chicken IGF-I was iodinated to a specific radioactivity of 150-300 Ci/g with the isotope I125, using a modified version of the chloramin-T (Kodak, Grand Island, NY, USA) technique (Lee and Henricks, 1990). IGF-I immunoreactivity was determined via the techniques developed by Lee et al. (1991). All assays were conducted in duplicate. The intra- and inter-assay coefficients of variation for IGFs were 8% and 10%, respectively.
Western ligand blot (WLB)
Samples underwent electrophoresis via 12% SDS-PAGE under non-reducing conditions using a Mini-Protein II system (Bio-Rad Laboratories, California, USA), and separated proteins were electro-blotted onto nitrocellulose membranes (Schleicher & Schuell Co, Dassel, Germany), as described by Hossenlopp et al. (1990). The membrane was then blocked with 1% BSA and incubated for 18 h at 4ºC with 1.5 x106 cpm of 125I-IGF-I in buffer containing 1% BSA and 0.1% Tween 20. After extensive washing in cold buffer, the membrane was dried at room temperature. Finally, it was exposed to X-ray film (RX medical film, Fuji Photo Film Co, Tokyo, Japan) for 7 days at 170ºC.
Samples underwent electrophoresis via 12% SDS-PAGE under non-reducing conditions using a Mini-Protein II system (Bio-Rad Laboratories, California, USA), and separated proteins were electro-blotted onto nitrocellulose membranes (Schleicher & Schuell Co, Dassel, Germany), as described by Hossenlopp et al. (1990). The membrane was then blocked with 1% BSA and incubated for 18 h at 4ºC with 1.5 x106 cpm of 125I-IGF-I in buffer containing 1% BSA and 0.1% Tween 20. After extensive washing in cold buffer, the membrane was dried at room temperature. Finally, it was exposed to X-ray film (RX medical film, Fuji Photo Film Co, Tokyo, Japan) for 7 days at 170ºC.
Western immunoblotting
The current experiment applied the Western immunoblotting method designed by Matstumoto et al. (1996), in which the acquired protein samples (20-30 mg) were separated on 10% SDS-polyacrylamide electrophoresis gels. Following electrophoresis, the protein samples were transferred to PVDF membranes (BIO-Rad, Hercules, CA, USA) and washed with 25 mM Tris-buffer (pH7.4), 137 mM NaCl and 0.1% Tween-20 buffer, then subsequently blocked using the same buffer containing 5% nonfat dried milk for 2 h at room temperature. The prepared sample blots were initially incubated overnight with antibodies against IGPBP-3 and IGFBP-1 at 4°C, and treated with goat or rat antirabbit horseradish peroxidase. After this treatment, the blots were washed and developed using ECL (Pierce Chemical, Rockford, IL, USA), and then exposed to X-ray film in an effort to detect protein bands.
1- Provided per kilogram of diet: vit. A, 5,500 IU; vit. D3, 1,100 IU; vit. E, 11 IU; vit. B12 0.0066 mg; riboflavin, 4.4 mg; niacin, 44 mg; pantothenic acid, 11 mg (Ca-pantothenate, 11.96 mg); choline, 190.96 mg (choline chloride 220 mg); menadione, 1.1 mg (menadione sodium bisulfite complex, 3.33 mg); folic acid, 0.55 mg; pyridoxine, 2.2 mg (pyridoxine hydrochloride, 2.67 mg); biotin, 0.11 mg; thiamin, 2.2 mg (thiamine mononitrate, 2.40 mg); ethoxyquin, 125 mg.
2- Provided in mg per kilogram of diet; MnSO4, 120; ZnSO4, 100; FeSO4, 60; CuSO4, 10; Ca (IO3)2, 0.46; CaCO3, min: 150 max: 180.
2- Provided in mg per kilogram of diet; MnSO4, 120; ZnSO4, 100; FeSO4, 60; CuSO4, 10; Ca (IO3)2, 0.46; CaCO3, min: 150 max: 180.
RT-PCR (reverse transcription-polymerase chain reaction)
Complementary DNA (cDNA) was prepared via reverse transcription of the total RNA from the laying hens. Total RNA (3
Complementary DNA (cDNA) was prepared via reverse transcription of the total RNA from the laying hens. Total RNA (3


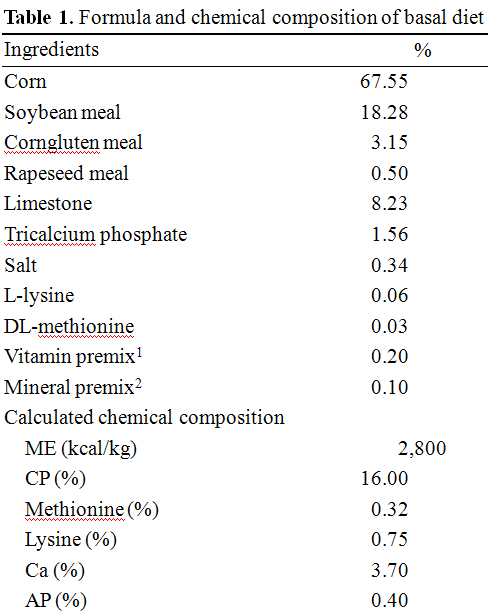
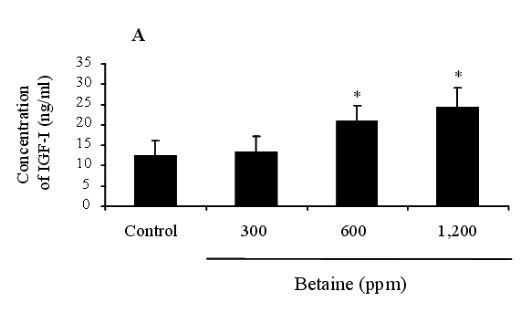
Figure 1. Effect of dietary betaine on the concentration of IGF-I in laying hens. The levels of concentration of IGF-I were determined by radioimmunoassay (RIA) in blood (A) and liver (B). Values were expressed as mean±SD (n = 6). * Significance was found compared to control (p<0.05).
Statistical analysis
The data obtained in the present investigation were analyzed via ANOVA and Student's t tests, and expressed as the meanRESULTS
The data obtained in the present investigation were analyzed via ANOVA and Student's t tests, and expressed as the meanRESULTS
Effect of betaine on the IGF-I concentration and gene expression in blood and liver
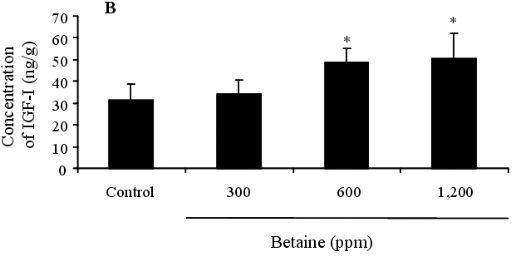
Figures 1 (A and B) illustrate the IGF-I concentrations in the blood and liver of feeding hens, after being fed on 0, 300, 600, and 1,200 ppm of betaine. The IGF-I concentration of the control recorded 12.453.67 ng/ml, whereas the betaine treatments increased IGF-I concentrations to levels of 13.244.13, 20.673.98, and 24.354.78 ng/ml, respectively. These elevated concentrations were statistically significant in the hens fed on 600 and 1,200 ppm of betaine (p<0.05) compared to the controls. The same pattern of IGF-I concentration increase relative to the control (31.237.45 ng/ml) was detected in the liver tissues of the hens at 34.206.57, 48.566.57, and 50.4511.78 ng/ml, respectively. In addition, a statistically significant increase in IGF-I gene expression was found in the birds fed on 600 ppm of betaine compared to the control, as illustrated in Figure 2 (A, B and C).
Figures 1 (A and B) illustrate the IGF-I concentrations in the blood and liver of feeding hens, after being fed on 0, 300, 600, and 1,200 ppm of betaine. The IGF-I concentration of the control recorded 12.453.67 ng/ml, whereas the betaine treatments increased IGF-I concentrations to levels of 13.244.13, 20.673.98, and 24.354.78 ng/ml, respectively. These elevated concentrations were statistically significant in the hens fed on 600 and 1,200 ppm of betaine (p<0.05) compared to the controls. The same pattern of IGF-I concentration increase relative to the control (31.237.45 ng/ml) was detected in the liver tissues of the hens at 34.206.57, 48.566.57, and 50.4511.78 ng/ml, respectively. In addition, a statistically significant increase in IGF-I gene expression was found in the birds fed on 600 ppm of betaine compared to the control, as illustrated in Figure 2 (A, B and C).
Effects of betaine on the identification of IGFBPs and concentration of IGFBP-1 and-3
The presence of IGFBPs in the blood was identified by Western ligand-blotting procedures. The results of this procedure allowed us to detect the presence of IGFBP-3, IGFBP-1, 2, and IGFBP-4 bands, and IGFBP-3 expression was identified in the blood of birds fed betaine (Figure 3A). Western immunoblotting (WIB) demonstrated a significant increase in the concentration of IGFBP-3 in the group treated with 600 ppm of betaine compared to the controls (Figure 3B and C). Using identical Western ligand-blotting techniques, the presence of IGFBPs in the livers of laying hens was identified in order to detect the IGFBP-3, IGFBP-1, 2, and IGFBP-4 bands. Among these IGFBPs bands, significant concentration variation of IGFBP-1 and 2 was found (Figure 3D). As shown in Figure 3 (E and F), the Western immunoblotting procedures revealed a significant reduction in IGFBP-1 secretion in 600 and 1,200 ppm betaine treatments compared to the controls.
The presence of IGFBPs in the blood was identified by Western ligand-blotting procedures. The results of this procedure allowed us to detect the presence of IGFBP-3, IGFBP-1, 2, and IGFBP-4 bands, and IGFBP-3 expression was identified in the blood of birds fed betaine (Figure 3A). Western immunoblotting (WIB) demonstrated a significant increase in the concentration of IGFBP-3 in the group treated with 600 ppm of betaine compared to the controls (Figure 3B and C). Using identical Western ligand-blotting techniques, the presence of IGFBPs in the livers of laying hens was identified in order to detect the IGFBP-3, IGFBP-1, 2, and IGFBP-4 bands. Among these IGFBPs bands, significant concentration variation of IGFBP-1 and 2 was found (Figure 3D). As shown in Figure 3 (E and F), the Western immunoblotting procedures revealed a significant reduction in IGFBP-1 secretion in 600 and 1,200 ppm betaine treatments compared to the controls.

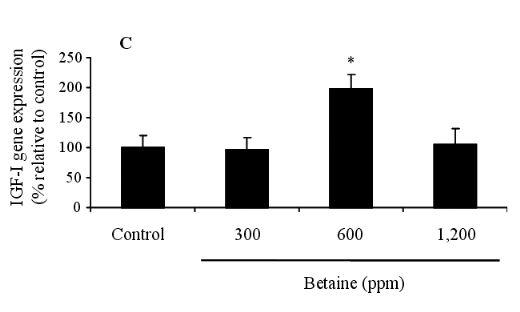
Figure 2. Effect of dietary betaine on the gene expression of IGF-I in liver of laying hens. The levels of gene expression (A) of IGF-I were determined by reverse transcriptase-polymerase chain reaction (RT-PCR). GAPDH was used as a loading control (B). The gene expression of IGF-I was conducted by densitometric analysis of the amplification products (C). Data represent percentages relative to control. Values were expressed as mean±SD (n = 6). * Significance was found compared to control (p<0.05).
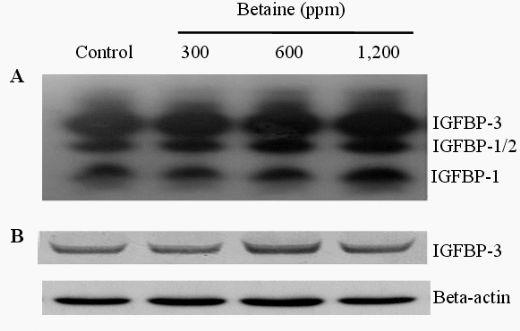
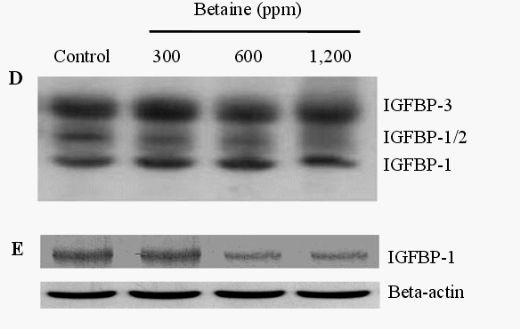
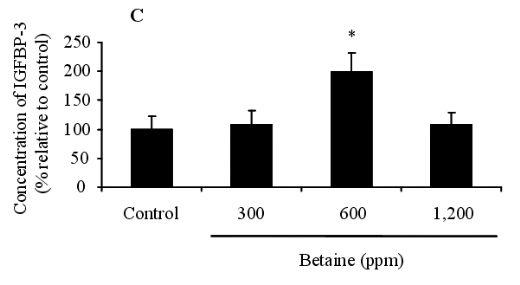
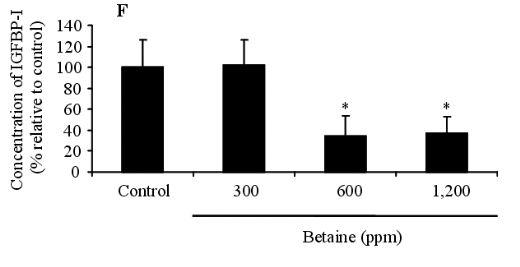
Figure 3. Effects of dietary betaine on the identification and concentration of IGFBPs in blood and liver of laying hens. The presence of IGFBPs was conducted by Western ligand-blotting in blood (A) and liver (D). The concentration of IGFBP-3 and IGFBP-1 were determined by western immunobloting with specific antibodies in the blood (B) and liver (E). The concentration of IGFBP-3 and IGFBP-1 was conducted by densitometric analysis of the western immunobloting (C and F). Beta-actin was used as loading controls. Data represent percentages relative to control. Values were expressed as mean±SD (n = 6). * Significance was found compared to control (p<0.05).
DISCUSSION
In addition to its functions as an osmolyte and methyl donor, betaine has also been shown to suppress the accumulation of fat, along with a variety of other nutritional, physiological, and pharmacological properties, which were previously reported by Saunderson and Mackinlay (1990). As a betaine metabolite (N, N, N-trimethyl glycine), glycine induces an increase in internal blood growth hormone levels, playing an essential role in the control of hypothalamic pituitary functions (Kasai et al., 1980). As it is synthesized by the mediation of a growth hormone (GH), IGF-I has also been reported to control directly the growth and differentiation of tissues via local secretion (Stewart and Rotwein, 1996). However, no detailed studies regarding the effects of a betaine-rich diet in laying hens on the regulation of IGFs in the blood and liver have previously been conducted. The primary objective of the present study was to systematically estimate the effects of a betaine diet on the IGF system in the blood and livers of laying hens. The results of this experiment showed IGF-I concentrations in the controls as 12.453.67 ng/ml and 31.237.45 ng/g in blood and liver, respectively. IGF-I concentration in the blood and liver revealed a pattern of increase depending on the betaine dose (p<0.05), and the same pattern was detected in the expression of mRNA in hepatocytes. It might be expected that the feedback stimulation of glycine, a betaine metabolite which consequently mediates blood GH secretion in the blood of laying hens, might constitute the cause of the promotion of IGF-I secretion. In addition, the increase in blood IGF-I levels might be attributed to the increase in liver IGF-I concentrations, as reported by Foresch et al. (1985), because 95% of blood IGF-I is synthesized by the liver. Such an increase in IGF-I mRNA expression as a relevant factor in IGF-I synthesis may have played a direct role in the increase in blood and liver IGF-I secretion. With regard to IGF-I binding proteins, also referred to as the Insulin-like growth factor-binding proteins (IGFBPs), 6 types have been identified and the majority of blood IGFBP-3 (up to 80%) is circulated in an IGF-I-bound form (Hill and Pell, 1998). As the principal roles of these binding proteins are to transport IGF-I to the target cells, they have also been reported to extend the half-life of IGF-I (Boxter, 1993; Rechler, 1993). As there is currently a lack of useful information regarding IGFBPs in laying hens, such previously reported functions of IGFBPs in other species could not be compared directly to laying hens. However, increased IGFBP-3 secretion in rodents and humans has been reported to affect the metabolism of estrogen secretion, immunity, and growth hormone increases (Salobir et al., 1996; Amy et al., 1998). Among these IGFBPs, IGFBP-1 is significantly increased by catabolic functions resulting from stress, infection, and immune deficiency, and increases in GH secretion and immunity suppress IGFBP-1 secretion (Lee et al., 1993; Jones and Clemmons, 1995). In the current study, increased secretion of IGFBP-3 was correlated with the amount of betaine fed to laying hens, but the secretion of IGFBP-1 was reduced in the livers of laying hens. This result may be attributed to the administration of a betaine diet to laying hens having induced an increase in the secretion of blood IGFBP-3, which consequently extended the half-life of blood IGF-I and increased preservability, enhancing the growth of laying hens and liver tissue differentiation. Via the above pathway, the consumption of betaine correspondingly suppressed IGFBP-1 secretion in the livers of laying hens as the result of the reduction in catabolic functions, which may have affected energy conservation. The current experiment demonstrated increased blood IGF-I concentration and IGF-I mRNA expression in the liver, but IGFBP-1 secretion in the liver was reduced. However, the increased secretion of IGF-I in the blood may have been induced by the increase in IGF-I mRNA expression in the livers of laying hens. The current study performed the crucial role in verifying the presence of IGF-1 and IGFBP-1 related to the results of Park et al. (2006) who previously reported the significant improvement of the performance of laying hens compared to controls when betaine was administered at the level of 600 ppm. Therefore, the results of this study could be employed in future investigations of the factors affecting the IGFs system of laying hens fed with betaine. Further study appears to be necessary in order to determine the systemic estimation of betaine associated with the productivity of laying hens.
REFERENCES
Amy, E. L., B. Patrice, H. C. Anne, A. C. Joseph and K. R. Michael. 1998. Regulation of experimental autoimmune encephalomyelitis with insulin-like growth factor (IGF-1) and IGF-1/IGF-binding protein-3 complex (IGF-1/IGFBP3). J. Clin. Invest. 101:1797-1804.
Barak, A. J. and D. J. Tuma. 1983. Betaine, metabolic by-product or vital. methylating agent?. Life Sci. 32:771-774.
Barak, A. J., H. C. Beckenhauer and D. J. Tuma. 1994. S-adenosylmethionine generation and prevention of alcoholic fatty liver by betaine. Alcohol 11:501-503.
Baxter, R. C. 1993. Circulating binding proteins for the insulin-like growth factors. Trends Endocrinol. Metab. 4:91-96.
Froesch, E. R., C. Schmid, J. Schwander and J. Zapf. 1985. Actions of insulin-like growth factors. Ann. Rev. Phys. 47:443-467.
Hill, R. A. and J. M. Pell. 1998. Regulation of insulin-like growth factor I (IGF-I) bioactivity in vivo: Further characterization of an IGF-I-enhancing antibody. Endocrinology 139:1278-1287.
Honarbakhsh, S., M. Zaghari and M. Shivazad. 2007. Can exogenous betaine be an effective osmolyte in broiler chicks under water salinity stress?. Asian-Aust. J. Anim. Sci. 20:1729-1737.
Hossenlopp, P., B. Segovia, C. Lassarre, M. Roghani, M. Bredon and M. Binoux. 1990. Evidence of enzymatic degradation of insulin-like growth factor-binding proteins in the 150k complex during pregnancy. J. Clin. Endocrinol. Metab. 71:797-805.
Jones, J. I. and D. R. Clemmons. 1995. Insulin-like growth factors and their binding proteins: biological actions. Endocr. Rev. 16:3-34.
Kasai, K., H. Suzuki, T. Nakamura, H. Shiina and S. I. Shimoda. 1980. Glycine stimulated growth hormone release in man. Acta Endocrinol. (Copenhagen) 93:283-286.
Kettunen, H., K. Tiihonen, S. Peuranen, M. T. Saarinen and J. C. Remus. 2001. Dietary betaine accumulates in the liver and intestinal tissue and stabilizes the intestinal epithelial structure in healthy and coccidian-infected broiler chicks. Comp. Biochem. Physiol. A 130:759-769.
Kidd, M. T., P. R. Ferket and J. D. Garlich. 1997. Nutritional and osmoregulatory functions of betaine. World's Poult. Sci. 53:125-139.
Lee, C. Y. and D. M. Henricks. 1990. Comparisions of various acidic treatments of bovine serum on insulin-like growth factor-I immunoreactive and binding activity. Endocrinology 127:139-148.
Lee, P. D. K., C. Conover and D. R. Powell. 1993. Regulation and function of insulin-like growth factor-binding protein-I. Proc. Soc. Exptl. Biol. Med. 204:4-29.
Lee, W. H., R. R. Bowsher, J. M. Apahy, M. C. Smith and D. P. Henry. 1991. Measurement of IGF-II in physiological fluid and tissues. II. Extraction in quantification in rat tissues. Endocrinology 128:815-822.
Matstumoto, T., S. E. Gargosky, K. Iwasaki and R. G. Resanfeld. 1996. Identification and charaterization of IGFs, IGFBPs and IGF proteases in human synovial fluid. J. Clin. Endocr. Metab. 81:150-155.
Nocianitri, K. A., S. Sakakibara, T. Kanno, H. Kikuchi, M.
Kurasaki and Y. Aoyama. 2002. Influence of dietary methionine level on the liver metallothionein mRNA level in rats. Biosci. Biotechnol. Biochem. 66:2465-2470.
Olivecrona, H., A. Hilding, C. Ekstrom, H. Barle, B. Nyberg, C. Moller, P. J. Delhanty, R. C. Baxter, B. Angelin, T. J. Ekstrom and M. Tally. 1999. Acute and short-term effects of growth hormone on insuline-like growth factors and their binding proteins: serum levels and hepatic messenger ribonucleic acid responses in humans. J. Clin. Endocr. Metab. 84:553-560.
Park, J. H., C. W. Kang and K. S. Ryu. 2006. Effects of feeding betaine on performance and blood hormone in laying hens. Kor. J. Poult. Sci. 33(4):323-328.
Rechler, M. M. 1993. Insulin-like growth factor binding proteins. Vitam. Horm. 47:1-114.Salobir, B., J. Prezelj, H. Meden-Vrtovec, A. Kocijancic and J. Osredkar. 1996. Insulin-like growth factor binding protein-3 (IGFBP-3) serum concentrations and ovarian responsiveness in in-vitro fertilization. Hum. Rep. 11:2208-2210.Saunderson, C. L. and J. MacKinlay. 1990. Changes in body-weight, composition and hepatic enzyme activities in response to dietary methionine, betaine and choline levels in growing chicks. Br. J. Nutr. 63:339-349.
Stewart, C. E. and P. Rotwein. 1996. Growth, differentiation, and survival: multiple physiological functions for insulin-like growth factors. Physiol. Rev. 76:1005-1026.
Zulkifli, I., S. A. Mysahra and L. Z. Jin. 2004. Dietary supplementation of betaine (betafin) and response to high temperature stress in male broiler chickens. Asian-Aust. J. Anim. Sci. 17:244-249.
Related topics:
Authors:
Chonbuk National University
Recommend
Comment
Share
Adisseo
6 de noviembre de 2010
Dear Keyong,
very informative technical study to understand role of Betaine to increase in internal Blood Growth Harmone levels leads to control of Hypthalamic Pituitary Functions.
Recommend
Reply
21 de octubre de 2010
A highly technical paper. We knew that natural Betaine is recommended to increase breast meat yield but in this paper, authors examine the growth at micro levels and now a field worker could fully understand how Betaine work in diet.
Recommend
Reply

Would you like to discuss another topic? Create a new post to engage with experts in the community.









