Prevalence, antimicrobial resistance and genetic diversity of Campylobacter coli and Campylobacter jejuni in Ecuadorian broilers at slaughter age
Author details:
Thermotolerant Campylobacter spp. are a major cause of foodborne gastrointestinal infections worldwide. The linkage of human campylobacteriosis and poultry has been widely described. In this study we aimed to investigate the prevalence, antimicrobial resistance and genetic diversity of C. coli and C. jejuni in broilers from Ecuador. Caecal content from 379 randomly selected broiler batches originating from 115 farms were collected from 6 slaughterhouses located in the province of Pichincha during 1 year. Microbiological isolation was performed by direct plating on mCCDA agar. Identification of Campylobacter species was done by PCR. Minimum inhibitory concentration (MIC) values for gentamicin, ciprofloxacin, nalidixic acid, tetracycline, streptomycin, and erythromycin were obtained. Genetic variation was assessed by RFLP-flaA typing and Multilocus Sequence Typing (MLST) of selected isolates. Prevalence at batch level was 64.1%. Of the positive batches 68.7% were positive for C. coli, 18.9% for C. jejuni, and 12.4% for C. coli and C. jejuni. Resistance rates above 67% were shown for tetracycline, ciprofloxacin, and nalidixic acid. The resistance pattern tetracycline, ciprofloxin, and nalidixic acid was the dominant one in both Campylobacter species. RFLPflaA typing analysis showed that C. coli and C. jejuni strains belonged to 38 and 26 profiles respectively. On the other hand MLST typing revealed that C. coli except one strain belonged to CC-828, while C. jejuni except 2 strains belonged to 12 assigned clonal complexes (CCs). Furthermore 4 new sequence types (STs) for both species were described, whereby 2 new STs for C. coli were based on new allele sequences. Further research is necessary to estimate the impact of the slaughter of Campylobacter positive broiler batches on the contamination level of carcasses in slaughterhouses and at retail in Ecuador.
Key words: Campylobacter, Ecuador, genetic types, antimicrobial resistance, broilers.
INTRODUCTION
MATERIALS AND METHODS
Study Design and Sampling
Isolation and Identification of Campylobacter spp.
Antimicrobial Resistance
Restriction Fragment Length Polymorphism of the flaA gene (flaA-RFLP)
Multilocus Sequence Typing
Statistical Analysis
RESULTS
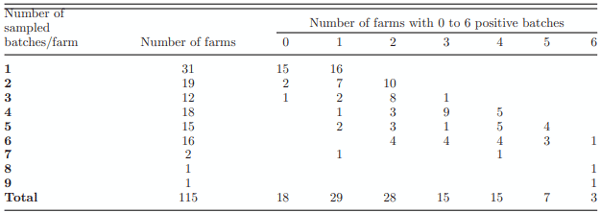
Prevalence of Campylobacter spp
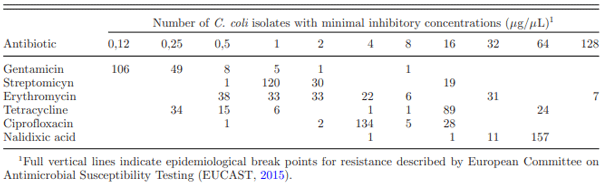
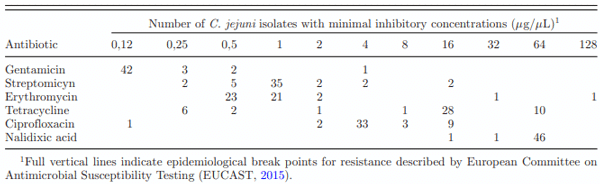
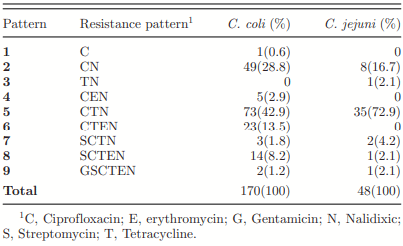
Antimicrobial Resistance
RFLP-flaA Typing
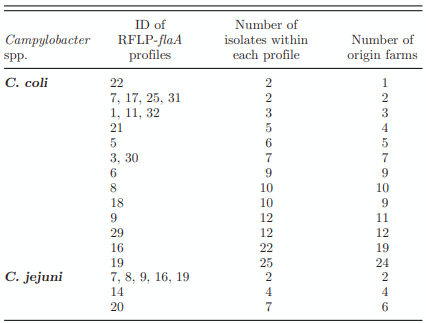
MLST Typing
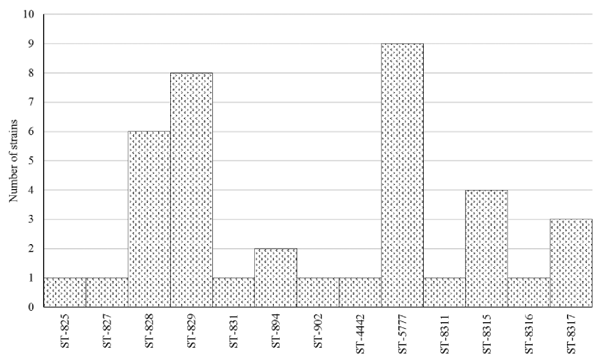
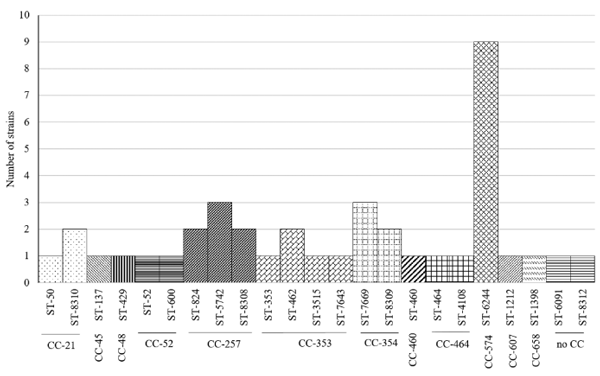
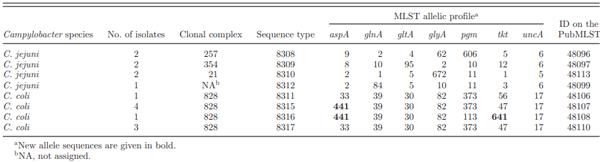
DISCUSSION
ACKNOWLEDGMENTS
Adkin, A., E. Hartnett, L. Jordan, D. Newell, and H. Davison. 2006. Use of a systematic review to assist the development of Campylobacter control strategies in broilers. J. Appl. Microbiol. 100:306– 315.
Carrique-Mas, J. J., J. E. Bryant, N. V Cuong, N. V. M. Hoang, J. Campbell, N. V Hoang, T. T. N. Dung, D. T. Duy, N. T. Hoa, C. Thompson, V. V Hien, V. V Phat, J. Farrar, and S. Baker. 2014. An epidemiological investigation of Campylobacter in pig and poultry farms in the Mekong delta of Vietnam. Epidemiol. Infect. 142:1425–1436. Available at http://www.pubmedcentral. nih.gov/articlerender.fcgi?artid=4045178&tool=pmcentrez& rendertype=abstract.
CGSIN and MAGAP. 2015. Registro Nacional de Producci´on Av´ıcola. Quito.
CONAVE. 2014. Estad´ısticas de Producci´on Av´ıcola 2013. 1.
Djordjevic, S. P., L. E. Unicomb, P. J. Adamson, L. Mickan, R. Rios, P. Adamson, K. Cheung, B. Combs, C. Dalton, R. Doyle, J. Ferguson, L. Gilbert, R. Givney, D. Gordon, J. Gregory, G. Hogg, T. Inglis, P. Jelfs, M. Kirk, K. Lalor, J. Lanser, L. O’Reilly, M. Sarna, H. Sharma, H. Smith, and M. Valcanis. 2007. Clonal complexes of Campylobacter jejuni identified by multilocus sequence typing are reliably predicted by restriction fragment length polymorphism analyses of the flaA gene. J. Clin. Microbiol. 45:102– 108.
Duarte, A., T. Seliwiorstow, W. G. Miller, L. De Zutter, M. Uyttendaele, K. Dierick, and N. Botteldoorn. 2016. Discriminative power of Campylobacter phenotypic and genotypic typing methods. J. Microbiol. Methods. 125:33–39. Available at http://linkinghub.elsevier.com/retrieve/pii/S0167701216300380.
Eberle, K. N., and A. S. Kiess. 2012. Phenotypic and genotypic methods for typing Campylobacter jejuni and Campylobacter coli in poultry. Poult. Sci. 91:255–264. Available at http://ps.oxfordjournals.org/content/91/1/255.full.
EFSA. 2010. Analysis of the baseline survey on the prevalence of Campylobacter n broiler batches and of Campylobacter and Salmonella on broiler carcasses in the EU, 2008, Part A: Campylobacter and Salmonella prevalence estimates. EFSA J. 15:100.
EFSA. 2011. Scientific Opinion on Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain. EFSA J. 9:2105. Available at http://doi.wiley.com/10.2903/j.efsa.2011.2105.
EFSA. 2015. SCIENTIFIC REPORT OF EFSA AND ECDC. EU Summary Report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2013. EFSA J. 13:1–178.
EFSA, and ECDC. 2015. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2014. EFSA J. 13:4329.
EUCAST. 2015. European committee on antimicrobial susceptibility testing. Data from the EUCAST MIC distribution website. Available at http://mic.eucast.org (verified 19 October 2016).
Ferro, I. D., T. M. Benetti, T. C. R. M. Oliveira, W. M. Abrah˜ao, S. M. S. S. Farah, F. B. Luciano, and R. E. F. Macedo. 2015. Evaluation of antimicrobial resistance of Campylobacter spp. isolated from broiler carcasses. Br. Poult. Sci. 56:66–71. Available at http://www.ncbi.nlm.nih.gov/pubmed/25567139.
Giombelli, A., and M. B. A. Gloria. 2014. Prevalence of salmonella and campylobacter on broiler chickens from farm to slaughter and efficiency of methods to remove visible fecal contamination. J. Food Prot. 77:1851–1859. Available at http://openurl.ingenta.com/content/xref?genre=article&issn= 0362-028X&volume=77&issue=11&spage=1851.
Graham, J. P., K. Vasco, and G. Trueba. 2016. Hyperendemic Campylobacter jejuni in guinea pigs (Cavia porcellus) raised for food in a semi-rural community of Quito, Ecuador. Environ. Microbiol. Rep. 8:382–387.
Griekspoor, P., E. O. Engvall, B. Olsen, and J. Waldenstrom. 2010. Multilocus sequence typing of Campylobacter jejuni from broilers. Vet. Microbiol. 140:180–185.
Havelaar, A. H., W. van Pelt, C. W. Ang, J. A. Wagenaar, J. P. M. van Putten, U. Gross, and D. G. Newell. 2009. Immunity to Campylobacter: its role in risk assessment and epidemiology. Crit. Rev. Microbiol. 35:1–22. Available at http://www.ncbi.nlm.nih.gov/pubmed/19514906.
Jonker, A., and J. A. Picard. 2010. Antimicrobial susceptibility in thermophilic Campylobacter species isolated from pigs and chickens in South Africa. J. S. Afr. Vet. Assoc. 81:228–236.
Jorgensen, F., J. Ellis-Iversen, S. Rushton, S. A. Bull, S. A. Harris, S. J. Bryan, A. Gonzalez, and T. J. Humphrey. 2011. Influence of season and geography on Campylobacter jejuni and C. coli subtypes in housed broiler flocks reared in Great Britain. Appl. Environ. Microbiol. 77:3741–3748.
Kittl, S., G. Heckel, B. M. Korczak, and P. Kuhnert. 2013. Source attribution of human Campylobacter isolates by MLST and Flatyping and association of genotypes with quinolone resistance. PLoS One. 8:1–8.
Levesque, S., E. Fournier, N. Carrier, E. Frost, R. D. Arbeit, and S. Michaud. 2013. Campylobacteriosis in urban versus rural areas: A case-case study integrated with molecular typing to validate risk factors and to attribute sources of infection. PLoS One. 8:17–20.
L´evesque, S., E. Frost, R. D. Arbeit, and S. Michaud. 2008. Multilocus sequence typing of Campylobacter jejuni isolates from humans, chickens, raw milk, and environmental water in Quebec, Canada. J. Clin. Microbiol. 46:3404–3411.
Loshaj-Shala, A., L. Regazzoni, A. Daci, M. Orioli, K. Brezovska, A. P. Panovska, G. Beretta, and L. Suturkova. 2015. Guillain Barr´e syndrome (GBS): new insights in the molecular mimicry between C. jejuni and human peripheral nerve (HPN) proteins. J. Neuroimmunol. 289:168–176. Available at http://www.ncbi.nlm.nih.gov/pubmed/26616887.
Ma, L., Y. Wang, J. Shen, Q. Zhang, and C. Wu. 2014. Tracking Campylobacter contamination along a broiler chicken production chain from the farm level to retail in China. Int. J. Food Microbiol. 181:77–84. Available at http://dx.doi.org/10.1016/j.ijfoodmicro.2014.04.023.
McDowell, S. W. J., F. D. Menzies, S. H. McBride, A. N. Oza, J. P. McKenna, A. W. Gordon, and S. D. Neill. 2008. Campylobacter spp. in conventional broiler flocks in Northern Ireland: Epidemiology and risk factors. Prev. Vet. Med. 84:261–276.
Nachamkin, I., K. Bohachick, and C. M. Patton. 1993. Flagellin gene typing of Campylobacter jejuni by restriction fragment length polymorphism analysis. J. Clin. Microbiol. 31:1531–1536. Available at http://www.ncbi.nlm.nih.gov/pubmed/8100241.
Ngulukun, S., S. Oboegbulem, and G. Klein. 2016. Multilocus sequence typing of Campylobacter jejuni and Campylobacter coli isolates from poultry, cattle and humans in Nigeria. J. Appl. Microbiol. Available at http://doi.wiley.com/10.1111/jam.13185.
O’Mahony, E., J. F. Buckley, D. Bolton, P. Whyte, and S. Fanning. 2011. Molecular epidemiology of campylobacter isolates from poultry production units in southern Ireland (T Kimman, Ed.). PLoS One 6:e28490. Available at http://dx.plos.org/10.1371/journal.pone.0028490.
Piccirillo, A., M. Giacomelli, C. Salata, S. Bettanello, E. De Canale, and G. Pal`u. 2014. Multilocus sequence typing of Campylobacter jejuni and Campylobacter coli from humans and chickens in North-Eastern Italy. 557–562.
Platts-Mills, J. A., and M. Kosek. 2014. Update on the burden of Campylobacter in developing countries. Curr. Opin. Infect. Dis. 27:444–450. Available at http://www.ncbi.nlm.nih.gov/pubmed/24655651.
Pollett, S., C. Rocha, R. Zerpa, L. Pati˜no, A. Valencia, M. Cami˜na, J. Guevara, M. Lopez, N. Chuquiray, E. Salazar-Lindo, C. Calampa, M. Casapia, R. Meza, M. Bernal, D. Tilley, M. Gregory, R. Maves, E. Hall, F. Jones, C. S. Arriola, M. Rosenbaum, J. Perez, and M. Kasper. 2012. Campylobacter antimicrobial resistance in Peru: a ten-year observational study. BMC Infect. Dis. 12:193. Available at http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid= 3482591&tool=pmcentrez&rendertype=abstract (verified 16 April 2013).
Prachantasena, S., P. Charununtakorn, S. Muangnoicharoen, L. Hankla, N. Techawal, P. Chaveerach, P. Tuitemwong, N. Chokesajjawatee, N. Williams, T. Humphrey, and T. Luangtongkum. 2016. Distribution and Genetic Profiles of Campylobacter in Commercial Broiler Production from Breeder to Slaughter in Thailand. PLoS One. 11:e0149585. Available at http://dx.plos.org/10.1371/journal.pone.0149585.
PubMLST.org. 2016. Campylobacter MLST Home Page. Available at PubMLST.org (verified 11 July 2016).
Reardon, S. 2014. Antibiotic resistance sweeping developing world. Nature. 509:141–142. Available at http://www.ncbi. nlm.nih.gov/pubmed/24805322 (verified 9 June 2014).
Rivera, N., R. Bustos, S. Montenegro H., M. Sandoval M., J. Castillo N., H. Fern´andez J., M. Maturana R., L. Delgado R, A. Contreras S., D. Ch´ ´ avez N, and I. Quevedo L. 2011. Genotipificaci´on y resistencia antibacteriana de cepas de Campylobacter spp aisladas en ni˜nos y en aves de corral. Rev. Chil. infectolog´ıa. 28:555–562. Available at http://www.scielo.cl/scielo.php?script=sci arttext&pid=S0716- 10182011000700008&lng=es&nrm=iso&tlng=es.
Sandberg, M., L. L. Sørensen, B. Steenberg, S. Chowdhury, A. K. Ersbøll, and L. Alban. 2015. Risk factors for Campylobacter colonization in Danish broiler flocks, 2010 to 2011. Poult. Sci. 94:447–453. Available at http://ps.oxfordjournals.org/content/94/3/447.abstract.
Seiffert, S. N., M. Hilty, V. Perreten, and A. Endimiani. 2013. Extended-spectrum cephalosporin-resistant Gram-negative organisms in livestock: an emerging problem for human health? Drug Resist. Updat. 16:22–45. Available at http://www.ncbi. nlm.nih.gov/pubmed/23395305 (verified 18 December 2014).
Sheppard, S. K., and M. C. J. Maiden. 2015. The Evolution of Campylobacter jejuni and Campylobacter coli. 1–14.
Sierra–Arguello, Y. M., G. Perdoncini, R. B. Morgan, C. T. P. Salle, H. L. S. Moraes, M. J. P. Gomes, and V. P. do Nascimento. 2016. Fluoroquinolone and Macrolide Resistance in Campylobacter Jejuni Isolated from Broiler Slaughterhouses in Southern Brazil. Avian Pathol. 9457:1–22. Available at http://www. tandfonline.com/doi/full/10.1080/03079457.2015.1120272.
Skarp, C. P. A., M.-L. H¨anninen, and H. I. K. Rautelin. 2015. Campylobacteriosis: the role of poultry-meat. Clin. Microbiol. Infect. 1–7. Available at http://linkinghub.elsevier.com/ retrieve/pii/S1198743X15010253.
Torralbo, A., C. Borge, A. Allepuz, I. Garc´ıa-Bocanegra, S. K. Sheppard, A. Perea, and A. Carbonero. 2014. Prevalence and risk factors of Campylobacter infection in broiler flocks from southern Spain. Prev. Vet. Med. 114:106–113. Available at http://dx.doi.org/10.1016/j.prevetmed.2014.01.019.
Tresierra-Ayala, A., H. Fern´andez, M. E. Benday´an, G. Pereyra, and A. Bernuy. 1995. Aislamiento de especies termotolerantes de Campylobacter en dos poblaciones de pollos criados con y sin confinamiento. Rev. Saude. Publica. 29:389–392. Available at http://www.scielo.br/scielo.php?script=sci arttext&pid=S0034- 89101995000500008&lng=es&nrm=iso&tlng=es.
Vandamme, P., L. J. Van Doorn, S. T. al Rashid, W. G. Quint, J. van der Plas, V. L. Chan, and S. L. On. 1997. Campylobacter hyoilei Alderton et al. 1995 and Campylobacter coli V´eron and Chatelain 1973 are subjective synonyms. Int. J. Syst. Bacteriol. 47:1055–1060. Available at http://www.ncbi.nlm.nih.gov/pubmed/9336905 (verified 28 July 2013).
Vasco, G., G. Trueba, R. Atherton, M. Calvopi˜na, W. Cevallos, T. Andrade, M. Eguiguren, and J. N. S. Eisenberg. 2014. Identifying etiological agents causing diarrhea in low income ecuadorian communities. Am. J. Trop. Med. Hyg. 91:563–569. Available at http://www.ncbi.nlm.nih.gov/pubmed/25048373 (verified 25 September 2014).
Wassenaar, T. M., and D. G. Newell. 2000. Genotyping of Campylobacter spp. Appl. Environ. Microbiol. 66:1–9.
WHO. 2011. Campylobacter. Fact sheet N◦255. Available at http://www.who.int/mediacentre/factsheets/fs255/en/ (verified 1 February 2016).
WHO. 2015. WHO Estimates of the Global Burden of Foodborne Diseases. First edit. World Health Organization, Geneva.
Zbrun, M. V, A. Romero-Scharpen, C. Olivero, E. Rossler, L. P. Soto, M. R. Rosmini, G. J. Sequeira, M. L. Signorini, and L. S. Frizzo. 2013a. Occurrence of thermotolerant Campylobacter spp. at different stages of the poultry meat supply chain in Argentina. N. Z. Vet. J. 61:337–343. Available at ://000325844500005.
Zbrun, M., A. Romero-Scharpen, C. Olivero, E. Rossler, L. Soto, M. Rosmini, G. Sequeira, M. Signorini, and L. Frizzo. 2013b. Occurrence of thermotolerant Campylobacter spp. at different stages of the poultry meat supply chain in Argentina. N. Z. Vet. J. Available at http://www.ncbi.nlm.nih.gov/pubmed/23906333 (verified 6 August 2013).
Zeng, D., X. Zhang, F. Xue, Y. Wang, L. Jiang, and Y. Jiang. 2016. Phenotypic characters and molecular epidemiology of campylobacter jejuni in East China. J. Food Sci. 81:M106–M113.
Zumbaco-Guti´errez, L., A. Ar´evalo-Madrigal, M. Donado-Godoy, and J. Romero-Z´uniga. 2014. DIAGNOSTICO MOLECULAR ´ DE Campylobacter EN LA Cadena. Agron. Mesoam. 25:357–363. Available at http://www.mag.go.cr/rev meso/v25n02 357.pdf.










.jpg&w=3840&q=75)