Introduction
Salmonella ranks among the world’s biggest threats to health. In the United States alone, it is responsible for around 580 deaths and an estimated 15,000 hospitalisations each year (WHO, 2005) - and these are just the reported cases. Annually, it has been estimated that cases of human salmonellosis in the United States may actually vary from 2 to 4 million (Jones, 2011). Salmonella is the second most prevalent cause of food poisoning in the US, where it is the primary bacterial food-borne disease (CDC, 2011) and receives significant attention from the World Health Organization (WHO) and the Centers for Disease Control. Developing and implementing effective Salmonella monitoring, reporting and control systems is prioritised in many countries. Salmonella is often associated with poultry products, mainly chicken and eggs. Salmonella are widely distributed in nature (Winfield and Groisman, 2003) and are able to survive for an extended period of time on diverse materials (Humphrey, 2004). Since its discovery in the late 19th Century, more than 2,500 different serovars have been discovered. All of these can cause disease in humans, which is most commonly associated with acute gastroenteritis – fever, abdominal pain, diarrhoea and nausea, sometimes with vomiting. If the pathogen enters the bloodstream or the disease leads to dehydration, effective antibiotic treatment is essential. Children and the elderly are particularly vulnerable, as are people with weakened immune systems. The Centers for Disease Control and Prevention recently estimated a total annual cost of US$3 billion associated with Salmonella (CDC, 2011). Similar calculations from Denmark suggest that spending the equivalent of US$14.1 million implementing a salmonella control programme could result in a net saving of US$25.5 million to the national economy (WHO, 2005).
Like other foodborne pathogens, Salmonella can develop resistance to antibiotics. Animal production has come under significant scrutiny in this regard. Preventing or treating diarrhoeal diseases in livestock has played a part in the development of multi-drug resistant strains. Salmonella enteritidis and S. typhimurium are the two serovars most commonly seen in human salmonellosis epidemics (Garber et al., 2003). These have emerged over the past 30 years, in parallel with the development of intensive systems of animal husbandry. In the European Union, the proportion of Salmonella and E. coli isolates resistant to ampicillin, sulfonamides and tetracycline were found to vary between 5 and 68 % in poultry, pigs and cattle. Some Member States reported a high occurrence of fluoroquinolone resistance in Salmonella isolates from poultry (5-38%), (EFSA, 2010).
The risks posed by contamination with pathogenic bacteria in the food chain can be reduced without the prophylactic use of antibiotics. Applying appropriate control measures at intervention points in the food chain can help reduce the risk of Salmonella proliferation. While Salmonella cannot be fully eradicated in poultry units, it can be controlled to minimise the risk to consumers. According to Jones (2011) Salmonella control measures in feed can be divided into three major categories: prevent contamination of the facility; measures to reduce multiplication of the bacteria in the plant; and procedures to kill the pathogen. Biosecurity plays a significant role in Salmonella control. In feed compounding, although heat treatment is effective in reducing contamination of feed leaving the feed mill, this effect does not persist during transport, storage and subsequent out-feeding. When conditions within the feed are less conducive to bacterial infection, Salmonella contamination can be reduced. The next critical control point is within the bird, where conditions for bacterial growth are optimal. Salmonella growth is optimal between 35 and 37°C, with moisture content greater than 12% and a pH of 4.5-9.0. Jones (2011) suggests addition of chemical agents to the feed to control Salmonella. This may primarily involve the use of organic acids.
Since the 1980’s, reports have shown organic acids, and formic acid in particular, to be especially effective against Salmonella, when used in poultry diets. The use of pure formic acid in breeder diets reduced the contamination of tray liners and hatchery waste with S. enteritidis drastically (Humphrey and Lanning, 1988). By 1990, researchers in the US found significantly reduced levels of Salmonella spp. in carcass and caecal samples, after including calcium formate in broiler diets (Izat et al., 1990). Further research (Kovarik and Lojda, 2000) reported that inclusion of formic acid at 0.5% in the diet can be successfully used on farms to reduce salmonella contamination in the feed, excretion of Salmonella spp. and re-infection of chicken populations.
A number of practical considerations also need to be addressed. Pure formic acid, although it is very effective in controlling Salmonella in feed, is corrosive, hazardous and volatile, so is difficult to handle easily and safely in the feed mill. Furthermore, pelleting may incur losses of around 15% of the acid. Often, liquid and volatile acids exert their antibacterial effects only in the feed and the birds’ foregut. More recently, research has focused on overcoming these limitations to develop chemical compounds which are heat-stable, non-corrosive and yet still effective. Diformates like sodium diformate (NDF) satisfy such industry requirements. An organic acid salt, it is crystalline and non-volatile, meaning that it can be used safely in the feed mill, as well as being effective in the animal.
Materials and methods
A recent study performed in the UK evaluated the anti-Salmonella effects in vitro, of a commercially available organic acid (sodium diformate, NDF) against Salmonella enteritidis (SE)S9549/07 found in broiler flocks (Wales et al., 2013). Caecal and crop samples were taken from slaughtered broilers from small-scale commercial operations. Caecal contents were used fresh; crop contents were stored at -80°C and thawed before use. Both were mixed with quarter strength Ringer’s solution (crop at a 1:1 ratio; caecal contents at 1:2). NDF was added to 20g aliquots in tubes. These were incubated in a water bath for 10 minutes at 41.5°C, after which time a 0.1ml stationary phase SE culture was added. All preparations were vortex mixed and incubated at 41.5°C. After various time intervals (1, 4 or 8 hours for crop contents; 1, 4, 9 and 24 hours for caecal contents), 5g aliquots were taken, mixed with buffered peptone water (BPW) and prepared for Salmonella enumeration. SE counts were recorded as a log reduction, compared to the negative control.
The objective of the second study was to evaluate the effect of NDF in broilers in vivo, on the control of bacterial contamination in the digestive tract in comparison to a negative control in-vivo (Lückstädt and Theobald, 2009). 1750 broilers were distributed in 14 batches of 125 birds each (5 batches per treatment; excluding control with 4 batches only). The broilers were fed the following program: starter diet for 21 days, grower diet for 18 days and finisher diet for 3 days only. Birds were treated with 0.3 and 0.6% NDF. After 39 days of treatment, before the finisher feed was given, 10 birds from each of the 3 treatments were taken for further microbial analysis, and were screened for Salmonella. The collected data were analysed with ANOVA by the StatisticsXL program. A P<0.05 value was considered to be a significant result.
Results and discussion
In vitro study
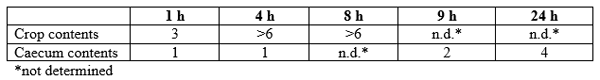
Table 1 shows the log reduction in SE counts after application of NDF at the manufacturer’s maximum recommended dose (0.6%) to samples of crop or caecal contents.
Table 1. Reduction in Salmonella enteritidis (log10) over time in crop or caecal content treated with 0.6% NDF (after Wales et al., 2013).
In the crop, exposure of inoculated crop contents to NDF resulted in a log 3 reduction in SE counts after 1 hour, reducing further to >log 6 at both 4 and 8 hours. Anti-Salmonella activity in the crop, by rapidly reducing the crop pH and killing Salmonella, may be particularly suited to combating the ingested pathogen from various contamination vectors (feed, environment, litter, etc.).
In caecal contents, only log 1 reduction in SE count was observed after 1 hour incubation, reducing further to log 2 reduction after 9 hours, compared to the negative control. This effect was further pronounced after 24 hours’ incubation, with a reduction in SE count of log 4. Since the retention time in the hind-gut of chickens is significantly longer, compared to the ‘foregut’ (crop, gizzard, proventriculus), the reduction in SE count after 24 hours may allow for a continuation of protection against the pathogen.
In vivo study
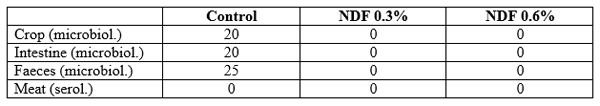
Results of the in vivo study are shown in Table 2 (Lückstädt and Theobald, 2009). No positive samples were found for Salmonella in the crop (P=0.15) or intestine (P=0.15) at either 0.3% (the recommended commercial dose in case of a suspected pathogenic challenge) or 0.6% (the maximum recommended dose, not used in practice).
Table 2. Results of various sodium diformate (NDF) dosages on Salmonella inhibition (% positive samples) in broiler chickens (after Lückstädt and Theobald, 2009).
The mode of action of the acidifier in poultry is mainly due to its antimicrobial action, unlike in pigs where a key activity is the reduction of stomach pH (Desai et al., 2007). These studies show that NDF achieves a rapid reduction in crop Salmonella count in vitro (Table 1), which is also found in vivo (Table 2).
Gram-negative pathogenic bacteria entering the bird’s intestinal tract can be effectively reduced in number by the addition of NDF to the feed. Sodium diformate is effective once it enters the gastrointestinal tract of the bird. Mixing with drinking water and gastric juices breaks one hydrogen bond, releasing a formate and formic acid, which are able to control pathogen load, either by reducing pH, as illustrated in the crop and gizzard/proventriculus, or exerting the direct antimicrobial effect of organic acids, which has been extensively documented previously. One of the main advantages of diformates in this regard, is the amount or organic acid/salts which enters the small intestine. In contrast to pure organic acids, diformates are able to deliver significant amounts of ‘acid material’ into the duodenum, as described by Mroz et al. (2000).
Using acidification in broiler diets is therefore a valuable strategy in a salmonella control programme. Diformates added to the feed exert their effects within the bird’s intestinal tract. Combined strategies, for example by including coarse feed particle size along with the use of diformate can have further beneficial impact in the fight against Salmonella (Visscher et al., 2009).
References
CDC – Centers for Disease Control, 2011. CDC estimates of foodborne illness in the United States. www.cdc.gov/foodborneburden (accessed February 2011).
EFSA, 2010. Community Summary Report: Antimicrobial resistance in zoonotic agents from animals and food in the European Union in 2004-2007.
Garber, L., Smeltzer, M., Fedorka-Cray, P., Ladely, S. and Ferris, K., 2003. Salmonella enterica Serotype enteritidis in table egg layer house environments and in mice in U.S. layer houses and associated risk factors. Avian Diseases 47: 134-142.
Humphrey, T., 2004. Salmonella, stress responses and food safety. Nat. Rev. Microbiol. 2: 504-509.
Humphrey, T., Lanning, D., 1988. The vertical transmission of salmonellas and formic acid treatment of chicken feed. A possible strategy for control. Epidemiology and Infection 100: 43-49.
Izat, A.L., Adams, M.H., Cabel, M.C., Colberg, M., Reiber, M.A., Skinner, J.T. and Waldroup, P.W., 1990. Effect of formic acid or calcium formate in feed on performance and microbiological characteristics of broilers. Poultry Science 69: 1876-1882.
Jones F., 2011. A review of practical Salmonella control measures in animal feed. J. Appl. Poult. Res. 20: 102-113.
Kovarik, K., Lojda, L.. 2000. WVPA News. www.wvpa.net/newsletter/reports_czech_rebubl.html.
Lückstädt, C., Theobald, P., 2009. Effect of a formic acid-sodium formate premixture on Salmonella, Campylobacter and further gut microbiota in broilers. Proceedings and Abstracts of the 17th European Symposium on Poultry Nutrition: 246.
Mroz, Z., Jongbloed, A.W., Øverland, M., 2000. Postprandial kinetics of supplemental K-diformate in duodenal digesta of weaned piglets. J. Anim. Sci. 78, suppl. 1: 193.
Visscher, C.F., Winter, P., Verspohl, J., Stratmann-Selke, J., Upmann, M., Beyerbach, M., Kamphues, J. 2009. Effects of feed particle size at dietary presence of added organic acids on caecal parameters and the prevalence of Salmonella in fattening pigs on farm and at slaughter. Journal of Animal Physiology and Animal Nutrition 93: 423-430.
Wales, A., McLaren, E., Rabie, A., Gosling, R.J., Martelli, F., Sayers, R., Davies, R., 2013. Assessment of the anti-Salmonella activity of commercial formulations of organic acid products. Avian Pathology 43(3): 268-275.
Winfield M., Groisman E., 2003. Role of non-host environments in the lifestyles of Salmonella and Escherichia coli. Applied Environmental Microbiology 69: 3687-3694.
World Health Organization, 2005. Drug-resistant Salmonella, Fact sheet No. 139, 5 pp.
*This paper was presented at the “International Congress on Advancements in Poultry Productions in the Middle East and African States”, Antalya, Turkey, 21-25 October 2013











.jpg&w=3840&q=75)