Tannins and Bacitracin Differentially Modulate Gut Microbiota of Broiler Chickens
Author details:
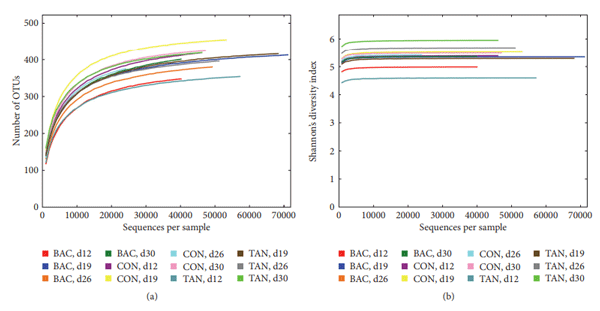
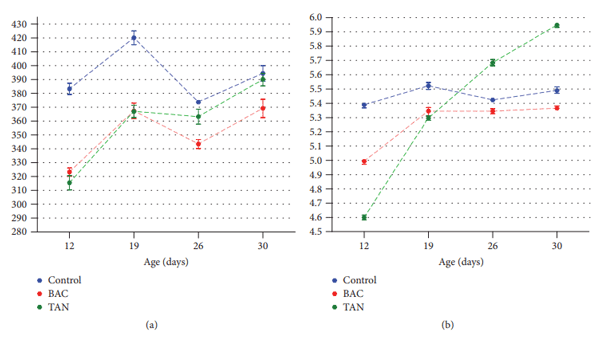
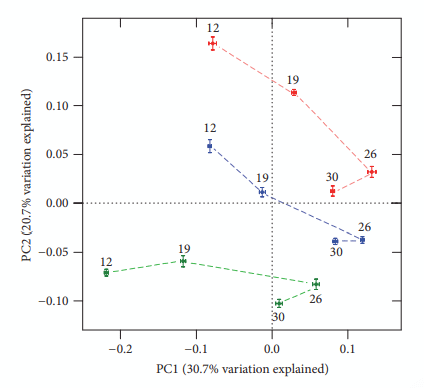
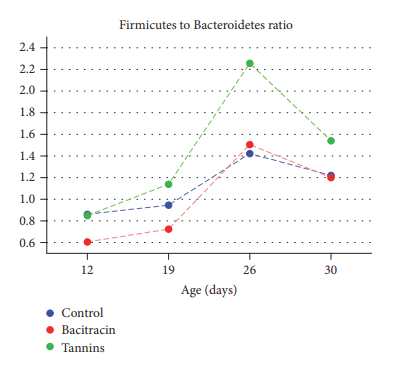
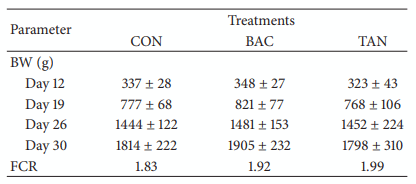
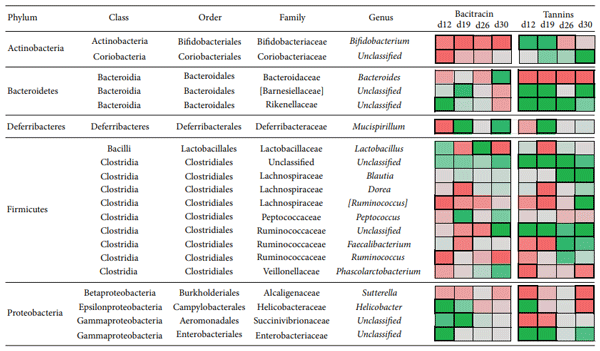
Antibiotic growth promoters have been used for decades in poultry farming as a tool to maintain bird health and improve growth performance. Global concern about the recurrent emergence and spreading of antimicrobial resistance is challenging the livestock producers to search for alternatives to feed added antibiotics. The use of phytogenic compounds appears as a feasible option due to their ability to emulate the bioactive properties of antibiotics. However, detailed description about the effects of in-feed antibiotics and alternative natural products on chicken intestinal microbiota is lacking. High-throughput sequencing of 16S rRNA gene was used to study composition of cecal microbiota in broiler chickens supplemented with either bacitracin or a blend of chestnut and quebracho tannins over a 30-day grow-out period. Both tannins and bacitracin had a significant impact on diversity of cecal microbiota. Bacitracin consistently decreased Bifidobacterium while other bacterial groups were affected only at certain times. Tannins-fed chickens showed a drastic decrease in genus Bacteroides while certain members of order Clostridiales mainly belonging to the families Ruminococcaceae and Lachnospiraceae were increased. Different members of these groups have been associated with an improvement of intestinal health and feed efficiency in poultry, suggesting that these bacteria could be associated with productive performance of birds.
[1] G. Huyghebaert, R. Ducatelle, and F. Van Immerseel, “An update on alternatives to antimicrobial growth promoters for broilers,” The Veterinary Journal, vol. 187, no. 2, pp. 182–188, 2011.
[2] B. M. Marshall and S. B. Levy, “Food animals and antimicro-bials: impacts on human health,” Clinical Microbiology Reviews, vol. 24, no. 4, pp. 718–733, 2011.
[3] D. F. Maron, T. J. S. Smith, and K. E. Nachman, “Restrictions on antimicrobial use in food animal production: an international regulatory and economic survey,” Globalization and Health, vol. 9, no. 1, article 48, 2013.
[4] H. K. Allen, U. Y. Levine, T. Loof t, M. Bandrick, and T. A. Casey, “Treatment, promotion, commotion: antibiotic alternatives in food-producing animals,” Trends in Microbiology, vol. 21, no. 3, pp. 114–119, 2013.
[5] S. H. Park, I. Hanning, A. Perrota, B. J. Bench, E. Alm, and S. C. Ricke, “Modifying the gastrointestinal ecology in alternatively raised poultry and the potential for molecular and metabolomic assessment,” Poultry Science, vol. 92, no. 2, pp. 546–561, 2013.
[6] H. Brussow,¨ “Growth promotion and gut microbiota: Insights from antibiotic use,” Environmental Microbiology, vol. 17, no. 7, pp. 2216–2227, 2015.
[7] E. Angelakis, “Weight gain by gut microbiota manipulation in productive animals,” Microbial Pathogenesis, vol. 106, pp. 162– 170, 2017.
[8] D. Stanley, R. J. Hughes, and R. J. Moore, “Microbiota of the chicken gastrointestinal tract: Influence on health, productivity and disease,” Applied Microbiology and Biotechnology, vol. 98, no. 10, pp. 4301–4310, 2014.
[9] D. Pan and Z. Yu, “Intestinal microbiome of poultry and its interaction with host and diet,” Gut Microbes, vol. 5, no. 1, pp. 108–119, 2014.
[10] G. C. Mead, “Microbes of the avian cecum: types present and substrates utilized,” Journal of Experimental Zoology, vol. 252, pp. 48–54, 1989.
[11] J. T. Brisbin, J. Gong, and S. Sharif, “Interactions between com-mensal bacteria and the gut-associated immune system of the chicken,” Animal Health Research Reviews, vol. 9, no. 1, pp. 101– 110, 2008.
[12] C. J. Yeoman, N. Chia, P. Jeraldo, M. Sipos, N. D. Goldenfeld, and B. A. White, “The microbiome of the chicken gastrointesti-nal tract,” Animal Health Research Reviews, vol. 13, no. 1, pp. 89– 99, 2012.
[13] B. B. Oakley, H. S. Lillehoj, M. H. Kogut et al., “T he chicken gastrointestinal microbiome,” FEMS Microbiology Letters, vol. 360, no. 2, pp. 100–112, 2014.
[14] S. K. Shapiro and W. B. Sarles, “Microorganisms in the intestinal tract of normal chickens,” Journal of Bacteriology, vol. 58, no. 4, pp. 531–544, 1949.
[15] L. L. Guan, K. E. Hagen, G. W. Tannock, D. R. Korver, G. M. Fasenko, and G. E. Allison, “Detection and identification of Lactobacillus species in crops of broilers of different ages by using PCR-denaturing gradient gel electrophoresis and amplified ribosomal DNA restriction analysis,” Applied and Environmental Microbiology, vol. 69, no. 11, pp. 6750–6757, 2003.
[16] M. G. Wise and G. R. Siragusa, “Quantitative analysis of the intestinal bacterial community in one- to three-week-old com-mercially reared broiler chickens fed conventional or antibiotic-free vegetable-based diets,” Journal of Applied Microbiology, vol. 102, no. 4, pp. 1138–1149, 2007.
[17] V. A. Torok, G. E. Allison, N. J. Percy, K. Ophel-Keller, and R. J. Hughes, “Influence of antimicrobial feed additives on broiler commensal posthatch gut microbiota development and perfor-mance,” Applied and Environmental Microbiology, vol. 77, no. 10, pp. 3380–3390, 2011.
[18] B. B. Oakley, R. J. Buhr, C. W. Ritz et al., “Successional changes in the chicken cecal microbiome during 42 days of growth are independent of organic acid feed additives,” BMC Veterinary Research, vol. 10, no. 1, article no. 282, 2014.
[19] M. J. Sergeant, C. Constantinidou, T. A. Cogan, M. R. Bedford, C. W. Penn, and M. J. Pallen, “Extensive microbial and func-tional diversity within the chicken cecal microbiome,” PLoS ONE, vol. 9, no. 3, Article ID e91941, 2014.
[20] D. Stanley, M. S. Geier, R. J. Hughes, S. E. Denman, and R. J. Moore, “Highly variable microbiota development in the chicken gastrointestinal tract,” PLoS ONE, vol. 8, no. 12, Article ID e84290, 2013.
[21] S. Wei, M. Morrison, and Z. Yu, “Bacterial census of poultry intestinal microbiome,” Poultry Science, vol. 92, no. 3, pp. 671– 683, 2013.
[22] J. Lu, U. Idris, B. Harmon, C. Hofacre, J. J. Maurer, and M. D. Lee, “Diversity and Succession of the Intestinal Bacterial Community of the Maturing Broiler Chicken,” Applied and Environmental Microbiology, vol. 69, no. 11, pp. 6816–6824, 2003.
[23] M. A. Mohd Shauf i, C. C. Sieo, C. W. Chong, H. M. Gan, and Y. W. Ho, “Deciphering chicken gut microbial dynamics based on high-throughput 16S rRNA metagenomics analyses,” Gut Pathogens, vol. 7, no. 1, article no. 4, 2015.
[24] J. Gong, W. Si, R. J. Forster et al., “16S rRNA gene-based analysis of mucosa-associated bacterial community and phylogeny in the chicken gastrointestinal tracts: From crops to ceca,” FEMS Microbiology Ecology, vol. 59, no. 1, pp. 147–157, 2007.
[25] J. H. Choi, G. B. Kim, and C. J. Cha, “Spatial heterogeneity and stability of bacterial community in the gastrointestinal tracts of broiler chickens,” Poultry Science, vol. 93, no. 8, pp. 1942–1950, 2014.
[26] D. Stanley, M. S. Geier, H. Chen, R. J. Hughes, and R. J. Moore, “Comparison of fecal and cecal microbiotas reveals qualitative similarities but quantitative differences,” BMC Microbiology, vol. 15, no. 1, article no. 51, 2015.
[27] P. Butaye, L. A. Devriese, and F. Haesebrouck, “Antimicrobial growth promoters used in animal feed: Ef fects of less well known antibiotics on gram-positive bacteria,” Clinical Microbi-ology Reviews, vol. 16, no. 2, pp. 175–188, 2003.
[28] G. Xie, G. C. Duff, L. W. Hall et al., “Alteration of digestive tract microbiome in neonatal Holstein bull calves by bacitracin methylene disalicylate treatment and scours,” Journal of Animal Science, vol. 91, no. 10, pp. 4984–4990, 2013.
[29] J. Lu, C. Hofacre, F. Smith, and M. D. Lee, “Effects of feed additives on the development on the ileal bacterial community of the broiler chicken,” Animal, vol. 2, no. 5, pp. 669–676, 2008.
[30] J. M. Diaz Carrasco, L. M. Redondo, E. A. Redondo, J. E. Dominguez, A. P. Chacana, and M. E. Fernandez Miyakawa, “Use of Plant Extracts as an Ef fective Manner to Control Clostridium perfringens Induced Necrotic Enteritis in Poultry,” BioMed Research International, vol. 2016, Article ID 3278359, 15 pages, 2016.
[31] J. Serrano, R. Puupponen-Pimia,¨ A. Dauer, A. Aura, and F. Saura-Calixto, “Tannins: current knowledge of food sources, intake, bioavailability and biological effects,” Molecular Nutri-tion & Food Research, vol. 53, no. 2, pp. 310–329, 2009.
[32] S. R. Hashemi and H. Davoodi, “Herbal plants and their deriv-atives as growth and health promoters in animal nutrition,” Veterinary Research Communications, vol. 35, no. 3, pp. 169–180, 2011.
[33] C. Yang, M. A. K. Chowdhury, Y. Huo, and J. Gong, “Phytogenic compounds as alternatives to in-feed antibiotics: potentials and challenges in application,” Pathogens, vol. 4, no. 1, pp. 137–156, 2015.
[34] Illumina Protocol: “16S Metagenomic Sequencing Library,” (2013).
[35] A. M. Bolger, M. Lohse, and B. Usadel, “Trimmomatic: a flexible trimmer for Illumina sequence data,” Bioinformatics, vol. 30, no. 15, pp. 2114–2120, 2014.
[36] T. Magocˇ and S. L. Salzberg, “FLASH: fast length adjustment of short reads to improve genome assemblies,” Bioinformatics, vol. 27, no. 21, pp. 2957–2963, 2011.
[37] R. C. Edgar, “Search and clustering orders of magnitude faster than BLAST,” Bioinformatics, vol. 26, no. 19, pp. 2460-2461, 2010.
[38] J. Kuczynski, J. Stombaugh, W. A. Walters, A. Gonzalez,´ J. G. Caporaso, and R. Knight, “Using QIIME to analyze 16S rRNA gene sequences from microbial communities,” Current Protocols in Microbiology, vol. Chapter 1, p. Unit 1E5, 2012.
[39] C. Lozupone, M. E. Lladser, D. Knights, J. Stombaugh, and R. Knight, “UniFrac: an effective distance metric for microbial community comparison,” The ISME Journal, vol. 5, no. 2, pp. 169–172, 2011.
[40] D. Parks and R. Beiko, “STAMP: Statistical Analysis of Metage-nomic Profiles,” in Encyclopedia of Metagenomics, pp. 1–6, Springer New York, New York, NY, USA, 2013.
[41] J. A. Patterson and K. M. Burkholder, “Application of prebiotics and probiotics in poultry production,” Poultry Science, vol. 82, no. 4, pp. 627–631, 2003.
[42] L. Wang, M. Lilburn, and Z. Yu, “Intestinal microbiota of broiler chickens as affected by litter management regimens,” Frontiers in Microbiology, vol. 7, article 593, 2016.
[43] J. Gong, H. Yu, T. Liu et al., “Effects of zinc bacitracin, bird age and access to range on bacterial microbiota in the ileum and caeca of broiler chickens,” Journal of Applied Microbiology, vol. 104, no. 5, pp. 1372–1382, 2008.
[44] J. L. Danzeisen, H. B. Kim, R. E. Isaacson, Z. J. Tu, and T. J. Johnson, “Modulations of the chicken cecal microbiome and metagenome in response to anticoccidial and growth promoter treatment,” PLoS ONE, vol. 6, no. 11, Article ID e27949, 2011.
[45] M. Pourabedin, L. Guan, and X. Zhao, “Xylo-oligosaccharides and virginiamycin differentially modulate gut microbial com-position in chickens,” Microbiome, vol. 3, no. 1, article no. 15, 2015.
[46] A. P. Neumann and G. Suen, “Differences in major bacterial populations in the intestines of mature broilers after feeding virginiamycin or bacitracin methylene disalicylate,” Journal of Applied Microbiology, vol. 119, no. 6, pp. 1515–1526, 2015.
[47] Y. O. Fasina, M. M. Newman, J. M. Stough, and M. R. Liles, “Effect of Clostridium perfringens infection and antibiotic administration on microbiota in the small intestine of broiler chickens,” Poultry Science, vol. 95, no. 2, pp. 247–260, 2016.
[48] A. A. Pedroso, J. F. M. Menten, M. R. Lambais, A. M. C. Racanicci, F. A. Longo, and J. O. B. Sorbara, “Intestinal bacterial community and growth performance of chickens fed diets containing antibiotics,” Poultry Science, vol. 85, no. 4, pp. 747– 752, 2006.
[49] A. Viveros, S. Chamorro, M. Pizarro, I. Arija, C. Centeno, and A. Brenes, “Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks,” Poultry Science, vol. 90, no. 3, pp. 566–578, 2011.
[50] J. Lin, A. A. Hunkapiller, A. C. Layton, Y.-J. Chang, and K. R. Robbins, “Response of intestinal microbiota to antibiotic growth promoters in Chickens,” Foodborne Pathogens and Disease, vol. 10, no. 4, pp. 331–337, 2013.
[51] T. J. Dumonceaux, J. E. Hill, S. M. Hemmingsen, and A. G. Van Kessel, “Characterization of intestinal microbiota and response to dietary virginiamycin supplementation in the broiler chicken,” Applied and Environmental Microbiology, vol. 72, no. 4, pp. 2815–2823, 2006.
[52] P. Singh, A. Karimi, K. Devendra, P. W. Waldroup, K. K. Cho, and Y. M. Kwon, “Influence of penicillin on microbial diversity of the cecal microbiota in broiler chickens,” Poultry Science, vol. 92, no. 1, pp. 272–276, 2013.
[53] D. Stanley, R. J. Hughes, M. S. Geier, and R. J. Moore, “Bacteria within the gastrointestinal tract microbiota correlated with improved growth and feed conversion: Challenges presented for the identification of performance enhancing probiotic bacteria,” Frontiers in Microbiology, vol. 7, article no. 187, 2016.
[54] R. E. Ley, R. Knight, and J. I. Gordon, “The human micro-biome: Eliminating the biomedical/environmental dichotomy in microbial ecology,” Environmental Microbiology, vol. 9, no. 1, pp. 3-4, 2007.
[55] P. J. Turnbaugh, R. E. Ley, M. A. Mahowald, V. Magrini, E. R. Mardis, and J. I. Gordon, “An obesity-associated gut micro-biome with increased capacity for energy harvest,” Nature, vol. 444, no. 7122, pp. 1027–1031, 2006.
[56] W. Zhao, Y. Wang, S. Liu et al., “T he dynamic distribution of porcine microbiota across different ages and gastrointestinal tract segments,” PLoS ONE, vol. 10, no. 2, Article ID e0117441, 2015.
[57] E. Jami, B. A. White, and I. Mizrahi, “Potential role of the bovine rumen microbiome in modulating milk composition and feed efficiency,”PLoS ONE, vol. 9, no. 1, Article ID e85423, 2014.
[58] D. Stanley, M. S. Geier, S. E. Denman et al., “Identification of chicken intestinal microbiota correlated with the efficiency of energy extraction from feed,” Veterinary Microbiology, vol. 164, no. 1-2, pp. 85–92, 2013.
[59] D. W. Waite and M. W. Taylor, “Characterizing the avian gut microbiota: Membership, driving influences, and potential function,” Frontiers in Microbiology, vol. 5, p. 223, 2014.
[60] G. G. Han, E. B. Kim, J. Lee et al., “Relationship between the microbiota in different sections of the gastrointestinal tract, and the body weight of broiler chickens,” SpringerPlus, vol. 5, no. 1, article no. 911, 2016.
[61] K. M. Singh, T. Shah, S. Deshpande et al., “High through put 16S rRNA gene-based pyrosequencing analysis of the fecal microbiota of high FCR and low FCR broiler growers.,” Molec-ular Biology Reports, vol. 39, no. 12, pp. 10595–10602, 2012.
[62] V. A. Torok, R. J. Hughes, L. L. Mikkelsen et al., “Identifica-tion and characterization of potential performance-related gut microbiotas in broiler chickens across various feeding trials,” Applied and Environmental Microbiology, vol. 77, no. 17, pp. 5868–5878, 2011.
[63] D. Stanley, S. E. Denman, R. J. Hughes et al., “Intestinal micro-biota associated with differential feed conversion efficiency in chickens,” Applied Microbiology and Biotechnology, vol. 96, no. 5, pp. 1361–1369, 2012.
[64] S. M. L. Kabir, “The role of probiotics in the poultry industry,” International Journal of Molecular Sciences, vol. 10, no. 8, pp. 3531–3546, 2009.
[65] E. Angelakis and D. Raoult, “The increase of Lactobacillus species in the gut flora of newborn broiler chicks and ducks is associated with weight gain,” PLoS ONE, vol. 5, no. 5, Article ID e10463, 2010.
[66] B. Baurhoo, P. R. Ferket, and X. Zhao, “Effects of diets containing different concentrations of mannanoligosaccharide or antibiotics on growth performance, intestinal development, cecal and litter microbial populations, and carcass parameters of broilers,” Poultry Science, vol. 88, no. 11, pp. 2262–2272, 2009.
[67] H. U. Rehman, W. Vahjen, W. A. Awad, and J. Zentek, “Indigenous bacteria and bacterial metabolic products in the gastrointestinal tract of broiler chickens,” Archives of Animal Nutrition, vol. 61, no. 5, pp. 319–335, 2007.
[68] F. Van Immerseel, F. Boyen, I. Gantois et al., “Supplementation of coated butyric acid in the feed reduces colonization and shedding of Salmonella in poultry,” Poultry Science, vol. 84, no. 12, pp. 1851–1856, 2005.
[69] C. L. Kien, R. Blauwiekel, J. Y. Bunn, T. L. Jetton, W. L. Frankel, and J. J. Holst, “Cecal infusion of butyrate increases intestinal cell proliferation in piglets,” Journal of Nutrition, vol. 137, no. 4, pp. 916–922, 2007.
[70] A. Biddle, L. Stewart, J. Blanchard, and S. Leschine, “Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities,” Diversity, vol. 5, no. 3, pp. 627–640, 2013.
[71] P. T. N. Lan, M. Sakamoto, S. Sakata, and Y. Benno, “Bacteri-odes barnesiae sp. nov., Bacteroides salanitronis sp. nov. and Bacteroides gallinarum sp. nov., isolated from chicken caecum,” International Journal of Systematic and Evolutionary Microbiol-ogy, vol. 56, no. 12, pp. 2853–2859, 2006.
[72] G. den Besten, K. van Eunen, A. K. Groen, K. Venema, D.-J. Reijngoud, and B. M. Bakker, “T he role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism,” Journal of Lipid Research, vol. 54, no. 9, pp. 2325– 2340, 2013.
[73] H. C. Lee, A. M. Jenner, C. S. Low, and Y. K. Lee, “Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota,” Research in Microbiology, vol. 157, no. 9, pp. 876–884, 2006.
[74] F. Cardona, C. Andres´-Lacueva, S. Tulipani, F. J. Tinahones, and M. I. Queipo-Ortuno,˜ “Benefits of polyphenols on gut microbiota and implications in human health,” The Journal of Nutritional Biochemistry, vol. 24, no. 8, pp. 1415–1422, 2013.
[75] H. Daniel, A. M. Gholami, D. Berry et al., “High-fat diet alters gut microbiota physiology in mice,” The ISME Journal, vol. 8, no. 2, pp. 295–308, 2014.
[76] J. R. E. Downward, N. R. Falkowski, K. L. Mason, R. Muraglia, and G. B. Huffnagle, “Modulation of post-antibiotic bacterial community reassembly and host response by Candida albicans,” Scientific Reports, vol. 3, article no. 2191, 2013.
[77] S. F. Clarke, E. F. Murphy, O. O’Sullivan et al., “Targeting the Microbiota to Address Diet-Induced Obesity: A Time Depen-dent Challenge,” PLoS ONE, vol. 8, no. 6, Article ID e65790, 2013.
[78] L. R. Lopetuso, F. Scaldaferri, V. Petito, and A. Gasbarrini, “Commensal Clostridia: leading players in the maintenance of gut homeostasis,” Gut Pathogens, vol. 5, no. 1, article 23, 2013.
[79] V. Eeckhaut, F. van Immerseel, S. Croubels et al., “Butyrate production in phylogenetically diverse Firmicutes isolated from the chicken caecum,” Microbial Biotechnology, vol. 4, no. 4, pp. 503–512, 2011.
[80] Z. H. Yuan, J. P. Wang, K. Y. Zhang et al., “Effect of Vanadium and Tea Polyphenols on Intestinal Morphology, Microflora and Short-Chain Fatty Acid Profile of Laying Hens,” Biological Trace Element Research, vol. 174, no. 2, pp. 419–427, 2016.
[81] T. Maˇsek, K. Starceviˇc,´ and Z. Mikulec, “The influence of the addition of thymol, tannic acid or gallic acid to broiler diet on growth performance, serum malondialdehyde value and cecal fermentation,” European Poultry Science, vol. 78, pp. 1–8, 2014.













