CLINICAL AND SUBCLINICAL DISEASE
Necrotic enteritis (NE) due to Clostridium perfringens is a disease of chickens (broilers, broiler breeders, commercial layers) and turkeys (Opengart and Songer, 2013). NE most often is diagnosed when it results in mortality and the presence of the gross lesion of a roughened-appearing mucosal surface of the small intestine at necropsy. However, the greatest economic loss to poultry producers may be due to the subclinical form of NE, which causes increased feed conversion and reduced growth rate.
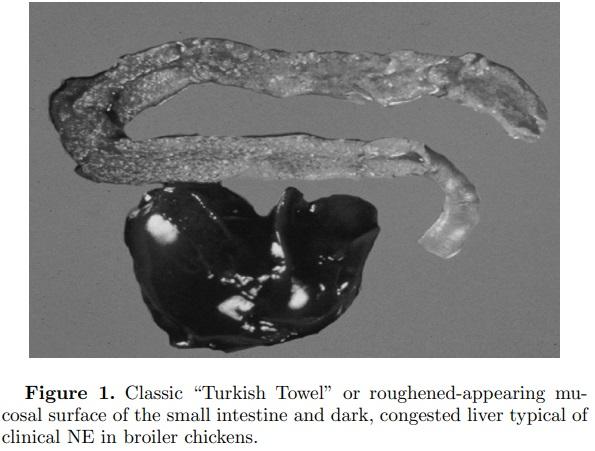
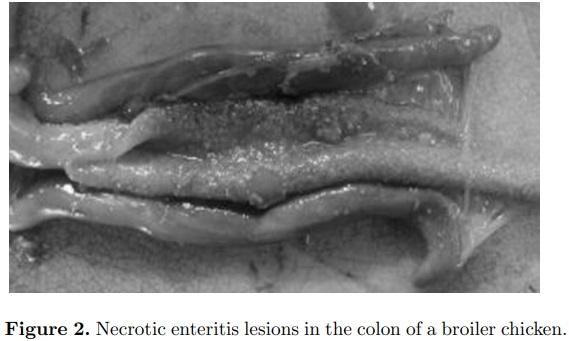
NE in broiler chickens is a common disease in most commercial poultry-growing regions of the world. It is seen primarily in broiler chickens between 10 and 28 d of age. The clinical form of the disease is also occasionally seen in broiler breeder pullets and adults, in commercial layer pullets and adults, and in turkeys (Braunius and Litjens, 1984; Broussard et al., 1986; Frame and Bickford, 1986; Droual et al., 1994; Dhillon et al., 2004). NE can present in 3 forms—peracute, acute, and subclinical. The peracute form of the disease is characterized by sudden elevation in mortality, sometimes without premonitory signs, whereas in the acute form birds may exhibit ruffled feathers, huddling (likely due to fever), and depression prior to death. In most instances there is a reduction in feed intake. The liver is frequently dark in color, swollen, firm, and turgid with a distended gall bladder. In some cases of NE, necrotic foci occur in the liver, which has been referred to as cholangiohepatitis (Wilson et al., 2005). This liver condition is more often seen when birds are fed a diet containing higher levels of small cereals such as wheat or barley. The most extreme NE lesion is typified by enlarged or ballooned small intestines with tan to brownish-colored diphtheritic pseudomembranes and copious watery brown blood-tinged fluid. There is often a foul odor upon opening the intestines. More typical is the classic “Turkish Towel” or roughened-appearing mucosal surface of the small intestine (Figure 1). Affected intestines are very friable or easily broken at necropsy. Necrotic enteritis lesions are most often observed in the descending loop of the duodenum into the jejunum and occasionally in the ileum. However, the authors have observed typical NE lesions of roughened necrotic mucosa in the colon (Figure 2).
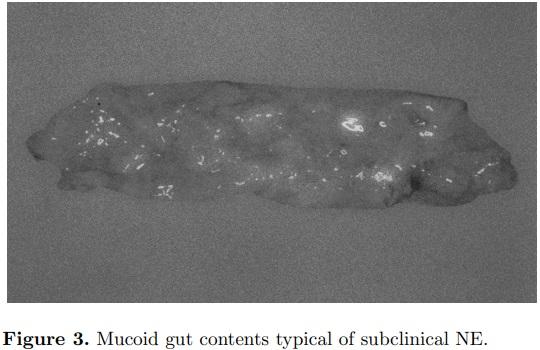
Subclinical NE, characterized by mucoid enteritis at necropsy, may have a greater economic impact for poultry producers than the clinical disease (Figure 3). Feed cost is from 65 to 75% of the cost to produce a broiler chicken. Therefore, anything that reduces the efficiency of the bird to convert feed into muscle will have a tremendous impact on the profitability for that producer. For the average U.S. broiler company slaughtering 1.6 million 3.25-kg birds per wk, an increase in feed conversion ratio from 1.66 to 1.70 would increase costs by US$80,000 per wk or annually US$4.16 million. Thus, subclinical Clostridium perfringens infection resulting in slightly increased mucus in the small intestines with impairment of digestion and nutrient absorption can be very costly. Subclinical NE has been estimated to cost the worldwide poultry industry billions of dollars each year (Van Der Sluis, 2000).
NE and the typical necrotizing enteritis observed in replacement pullets and adult broiler breeders often consists of focal or discrete necrotic areas of the small intestine rather than a diffuse pseudo membrane (Broussard et al., 1986). Classic NE has been observed in commercial turkeys but much less frequently than in broilers (Droual et al., 1994). In turkeys, it is usually observed between the ages of 7 to 12 wk, and more often is an extremely heavy mucoid enteritis.
AN OLD DISEASE RETURNS
A 2012 survey of broiler veterinarians in the southeastern region of the United States identified coccidiosis as the most significant broiler health problem and NE as second most significant (Hofacre, 2012). This trend is not unlike that reported in many other poultry-producing countries. The most common cause for intestinal injury that results in NE in commercial broilers is coccidial infections (Collier et al., 2008). As the coccidia complete their life cycle, they cause significant damage to the intestinal epithelial cells, resulting in increased mucus production and exponential growth of the C. perfringens. This intestinal damage and increased mucus production in subclinical NE is the reason for the estimated decline in feed conversation ratio of 4 points (Smith, 2011).
This increased incidence of coccidiosis and subsequent NE may be attributed to 2 situations globally. The primary means for control of coccidiosis since the early 1970s has been the ionophore anticoccidial drugs (McDougald, 1981). There have been no new ionophore anticoccidial drugs approved in the US for poultry since 1995 so there has been a significant increase in strains resistant to the ionophores (McDougald, 1982). Therefore, there is greater intestinal damage and more opportunity for C. perfringens to cause NE.
The second possible reason for an increase in a disease that was previously well controlled has been either the legislated or voluntary removal of the antibiotic growth-promotant drugs (AGP). In the EU, the AGPs were removed from the market by authorities, whereas in the U.S., there has been a market demand for poultry meat that has been raised free of all antibiotics (ABF), including the ionophore coccidiostats. In both situations, there has been a decline in the feed conversion of broiler chickens, most probably due to subclinical necrotic enteritis (Smith, 2011).
EXPERIENCE WITHOUT ANTIBIOTIC GROWTH-PROMOTANT DRUGS
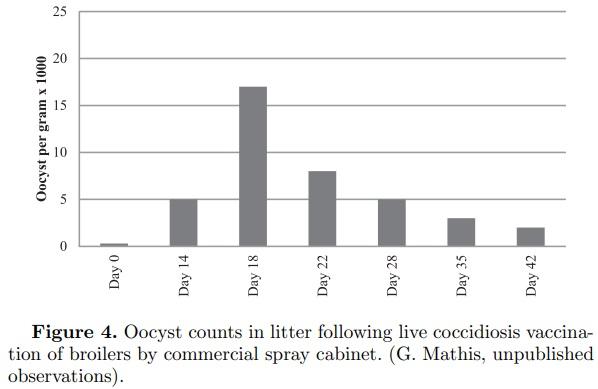
As resistance to the ionophore anticoccidials has increased, or as markets moved toward ABF production, there has been a greater use of live coccidiosis vaccines to control coccidiosis (Hofacre, 2012). These vaccines are composed of live coccidia that are either non-altered or slightly attenuated. Due to the nature of live vaccines, coccidia will develop in the cellular lining of the intestine, as does the wild type. This leads to varying degrees of intestinal cellular destruction. This cellular destruction and associated host responses can result in an increased proliferation of clostridium and an increased incidence of NE (Collier et al., 2008). A review of 372 cases of NE in one broiler company in the U.S. using coccidiosis vaccines without antibiotics found the earliest case occurred at 11 d and the latest was at 28 d of age with the median age of NE at 16.3 d (Smith, 2011). In 2001, Mathis et al. measured the oocyst counts in litter of broilers administered a commercial coccidiosis vaccine at 1 d of age by spray vaccination and found that the peak oocyst counts occurred on d 18 (Figure 4, G. Mathis, unpublished observations). Since release of oocysts requires intestinal damage, it would be reasonable to assume that birds not routinely fed an antibiotic would be at greater risk for NE at just prior to d 18 as observed by Smith at 16.3 d of age (Smith, 2011; McDougald and Fitz-Coy, 2013).
Other risk factors associated with an increased incidence of NE were removal of the litter between flocks, colder months (reduced ventilation/increased litter moisture), and overall poorer farmer husbandry. The incidence of NE in the winter may at times be as high as 30 to 50% of the houses placed (Smith, 2011).
There is also a need to improve the quality and sanitation of the hatching eggs that are produced for an ABF program. As the newly laid egg cools in a nest, the internal contents shrink, resulting in air and bacteria on the shell being pulled into the egg pores; motile bacteria in particular can penetrate the egg (Ernst et al., 1998; De Reu et al., 2006; Gantois et al., 2009). Antibiotics injected in ovo or at 1 d of age may help control the resulting neonatal infections (Vernimb et al., 1976). Thus, we observe an increase of wk 1 mortality of 0.25% to 0.50% or more in ABF programs compared to non-ABF.
PREVENTING NECROTIC ENTEROCOLITIS
Success in preventing both clinical and subclinical NE requires an understanding that intestinal epithelial damage must be reduced, proliferation of toxigenic strains of C. perfringens prevented, and a healthy intestinal microflora maintained.
Intestinal Epithelium Damage
Although there are many causes for damage to the small intestine mucosa (mycotoxins, ascarids, enteric viruses, rancid fats, biogenic amines, etc.), the primary source for damage of the epithelium found in nearly all poultry houses are the Eimeria species. Since the primary location of NE is in the duodenum and jejunum, it would stand to reason that E. acervulina and E. maxima would be the primary Eimeria species causing NE. Mathis (2005) and Mathis and Hofacre (2005) administered E. acervulina alone (50,000 oocysts), E. maxima alone (5,000 oocysts), and a combination of both E. maxima (5,000 oocysts) and E. acervulina (50,000 oocysts) by oral gavage to broilers at 14 d of age in battery cages (Mathis, 2005; Mathis and Hofacre, 2005). On d 17, 18, and 19, C. perfringens (C.P. #6 at 1.0 × 108 CFU/chick) was orally gavaged as per the NE challenge method of Hofacre et al. (1998). Both Eimeria species caused NE mortality with E. maxima causing the greatest mortality at nearly 45% (Figure 5). When both E. acervulina and E. maxima were present, the NE mortality was less, at 18%.
Inhibition of Clostridium perfringens Growth
Organic acids, inorganic acids, and phytoceutical products have been demonstrated to directly inhibit the growth of C. perfringens (Ricke, 2003; Dahiya et al., 2006; Skrivanova et al., 2006; Allaart et al., 2013; Diaz-Sanchez et al., 2015; M’Sadeq et al., 2015). The proposed mechanism of action for the acids is by intracellular absorption of the undissociated acid, which then dissociates within the cytoplasm, resulting in a drop of intracellular pH, exhaustion of energy supplies by the proton pump, and cell death (Ricke, 2003), although other mechanisms may be operable depending on the exact organic acid (Ricke, 2003; Skrivanova et al., 2006). There are many phytoceutical compounds such as essential oils, terpenes, flavonoids, phenolics, and saponins with purported antimicrobial actions, including artemisinin, betaine, citric extracts and organic acids, Echinacea purpurea, gentian violet, mushrooms and their extracts, oregano (carvacrol), thyme (thymol), cloves (eugenol), mustard (allysothiocyanate), garlic (allicin), curcumin, piperin, turmeric, Persian lilac, bitter melon, green tea, cinnamon (cinnamaldehyde), capsaicin, Yucca schidigera extracts, eucalyptus, cabbage tree extracts, golden wattle tree extracts, seaweed extracts, marjoram, rosemary, sage, yarrow, hops, grape pomace, pennyroyal, and others (Peek and Landman, 2011; Diaz-Sanchez et al., 2015). The proposed mechanism of action varies with the compound (Peek and Landman, 2011; Diaz-Sanchez et al., 2015).
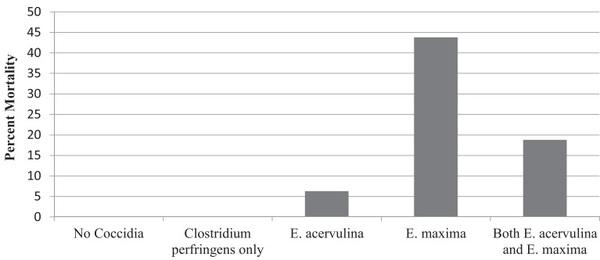
Figure 5. Necrotic enteritis mortality after challenge with E. acervulina alone, E. maxima alone, or both species together, followed in each case by inoculation with C. perfringens. (Mathis, 2005; Mathis and Hofacre, 2005).
Probiotics and prebiotics, by helping to maintain normal healthy flora, have been used to inhibit growth of Salmonella sp., Clostridium perfringens, and E. coli. The mechanism of action of probiotics is not completely understood but is thought to include competition with pathogens for nutrients and binding sites, production of inhibitory short-chain volatile fatty acids, alteration of gut pH, production of antimicrobial substances such as bacteriocins, and alteration of gut immune responses (M’Sadeq et al., 2015). The prebiotics are often starches that are non-digestible by the bird such as D-mannose or fructose, that selectively stimulate the growth or activity of beneficial normal gut flora (Dahiya et al., 2006; M’Sadeq et al., 2015), or are yeast cell wall components such as mannanoligosaccharides (often referred to as MOS) that bind bacterial fimbriae and act as pathogenassociated molecular patterns to stimulate the innate immune system (Peek and Landman, 2011). They also are reported to promote villus development (M’Sadeq et al., 2015). Replacing an antibiotic in an ABF program cannot be successfully accomplished by a single product or strategy. A combined program to minimize the disease caused by coccidia and keep C. perfringens from multiplying to high levels is necessary to achieve successful ABF production.
Alterations in the flora of the ceca can result in C. perfringens beginning to replicate and produce toxin (Collier et al., 2008; Timbermont et al., 2011). These toxins and C. perfringens are then carried up to the small intestines by the normal retroperistalsis of a chicken’s intestinal tract where the major damage occurs. Lu et al. demonstrated that feed ingredients such as wheat or feed additives such as antibiotics or ionophorous antibiotics can alter the ceca flora (Lu et al., 2008). These non-starch polysaccharides (NSPs) found in the small cereal grains such as wheat, barley, and rye increase the incidence of NE (Drew et al., 2004; McDevitt et al., 2006; Timbermont et al., 2011; Moran, 2014; M’Sadeq et al., 2015).
Food Safety Implications
ABF production may have an impact on the food safety of poultry products. Baba et al. (1987) found that disease by Eimeria tenella resulted in higher levels of S. typhimurium in spleens and livers, and Volkova et al. (2011) noted that the method of coccidiosis control may influence the prevalence of Salmonella at the processing plant (Baba et al., 1987; Volkova et al., 2011). The disease NE may not directly result in an increased colonization of Salmonella, but it can result in greater variation in bird sizes due to the subclinical form of the disease (Hofacre et al., 2007). As Russell (2003) found, poorer body weight uniformity (in his study, due to air sacculitis) can result in greater intestinal tract tears and greater risk of pathogen contamination on the carcass (Russell, 2003).
CONCLUSION
NE in both its clinical and subclinical forms has become a major health, welfare and performance condition as the poultry industry has reduced its use of antibiotics. Since NE is a multifactorial disease involving Eimeria sp. and the bacterium C. perfringens, control without antibiotics will require multiple methods of prevention and treatment. Removal of antibiotics from the birds’ diet should not be done solely for marketing purposes without provisions for antibiotic treatment of birds when a severe bacterial disease condition occurs, e.g., NE, E. coli airsacculitis, etc. Delayed treatment, or refusal to treat, in the face of serious mortality due to a treatable bacterial infection in order to maintain a marketing claim is immoral and unethical and a serious breach of animal welfare.
The poultry producers of the world have been extremely successful in providing poultry meat and eggs at a very wholesome and affordable price. These same producers will continue to supply the same product while using less antibiotics.
This article was originally published in 2018 Poultry Science 97:1929–1933. http://dx.doi.org/10.3382/ps/pey082. This is an Open Access article under a Creative Commons license.