Marek´s Disease
Molecular characteristics of meq oncogene from recently isolated Polish field strains of Marek’s disease virus
Published: October 19, 2011
By: Grzegorz Wozniakowski, Elżbieta Samorek-Salamonowicz, Wojciech Kozdruń (National Veterinary Research Institute)
Summary. The presented study was done on 29 MDV strains isolated from field cases of Marek´s disease in Poland. Molecular analysis performed on these strains by PCR and sequencing of meq oncogene has shown 68 bp insertion. This genetic change resulted in frame shift within 10 recently isolated field strains. These strains were tested by PCR for the detection of long-terminal repeat (LTR) region of reticuloendotheliosis virus (REV). The performed study have shown 0.78, 0.7, 0.82, 1.6 kb and other random inserts into MDV genome of examined Polish strains. These inserts were stable after 3 passages in chicken embryo kidney cells that suggests their stable integration with non-functional region of MDV genome. Further analysis of the new MDV strains revealed these inserts had no influence on the results of immunofluoroescence assay with FD7 anti-Meq antibodies.
Keywords. Marek´s disease, reticuloendotheliosis virus, recombination.
Introduction. Tumoral diseases of poultry are caused by Marek´s disease virus (MDV) and avian retroviruses causing lymphoma. MDV genomic and molecular structure are specific for Alphaherpesviriuses.The genome of MDV consist ofdouble stranded DNA about 180 kb which is separated into unique long (UL) and unique short (Us) regions flanked by internal (ITR) and terminal (TR) repeats. The main MDV´oncoprotein is Meq with leucine zipper motif (b-ZIP) specific for all transcriptional regulators. The gene is located between the junction of IRL and IRs and TRL region.
MDV genome is susceptible on recombination with other viral genomes especially retroviruses represented by reticuloendotheliosis virus (REV) and avian leukemia virus [2, 3, 5]. Retroviridae family include reticuloendotheliosis virus (REV) which fall into Gammaretrovirus genus while avian leucosis viruses (ALV) represent another Alpharetrovirus genus. The structure of retroviral genomes consist of single-stranded RNA with positive polarity. REV is one of an immunosuppressive factor affecting humoral and cellular immunity [12]. The virus is known to be present as a part of MDV´s DNA [2, 3, 6]. The possible influence of such genetic event may cause changes in properties of the viral proteins resulting raise of oncogenity but also attenuation. The aim of this study was to find the possible cause of observed changes within nucleotide sequence of meq.
MDV genome is susceptible on recombination with other viral genomes especially retroviruses represented by reticuloendotheliosis virus (REV) and avian leukemia virus [2, 3, 5]. Retroviridae family include reticuloendotheliosis virus (REV) which fall into Gammaretrovirus genus while avian leucosis viruses (ALV) represent another Alpharetrovirus genus. The structure of retroviral genomes consist of single-stranded RNA with positive polarity. REV is one of an immunosuppressive factor affecting humoral and cellular immunity [12]. The virus is known to be present as a part of MDV´s DNA [2, 3, 6]. The possible influence of such genetic event may cause changes in properties of the viral proteins resulting raise of oncogenity but also attenuation. The aim of this study was to find the possible cause of observed changes within nucleotide sequence of meq.
Materials and methods. Twenty-nine MDV field strains isolated from spleens of infected chickens. As the positive controls HPRS16 reference strain (obtained from dr Biggs, Houghton, U.K.), Rispens strain from commercial vaccine (Merial) and two BAC clones of Polish strains were used. All strains were propagated in chicken embryo kidney cells (CEK) prepared from SPF chicken embryos (LTZ, Germany). Third passage of each strain in CEKs was suspended in preservative medium containing DMSO 10% then gradually chilled and frozen in liquid nitrogen. Immunofluorescence assay (IFA) with FD7 anti-Meq antibodies was obtained from Dr Sue Baigent (IAH, Compton, Berkshire, UK) and conducted according to previously used procedure (1)
Total DNA was extracted directly from infected CEK cells using QIAamp Mini Kit (Qiagen) according to manufacturer´s requirements.
PCR was conducted for the amplification of meq oncogene and was described elsewhere [11]. PCR products were separated in 1.5% ethidium bromide stained agarose gels and were purified using Nucleospin Kit (Macherey-Nagel) PCR for LTR. The sequences of used primers specific for LTR-REV were described elsewhere (2). Reaction conditions were identical with the amplification of meq.
PCR products of meq from 24 MDV strains and 10 products for the LTR region were sequenced from forward and reversed primers on GS FLX/Titanium sequencer (Roche). The meq and LTR sequences were assembled and aligned using Genious ver. 5.1 [4].
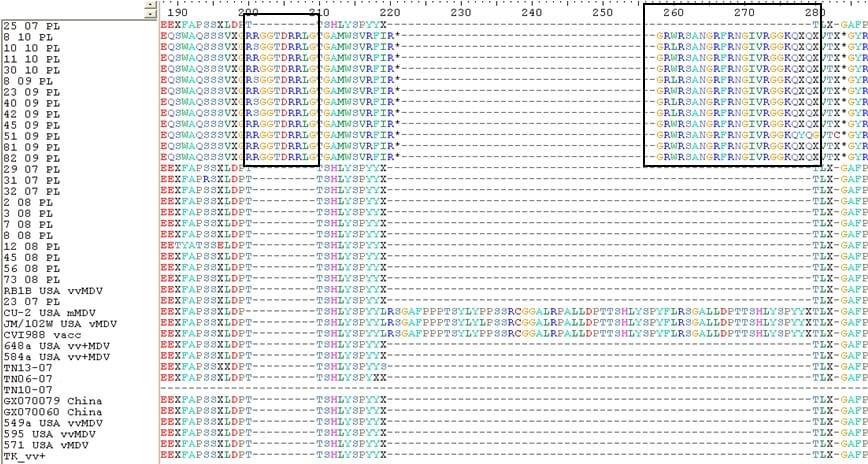
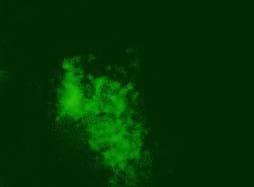
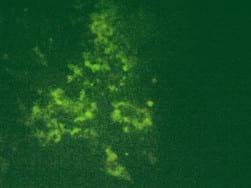
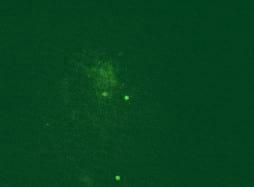
Results. CPE was observed in all infected cell cultures starting from the 96h of 3rd passage. Infected cells formed focuses and syncytias. The results obtained by IFA have shown no influence of the observed insertion of the obtained results (Fig.1). DNA extracted from the infected cell cultures was used for the further study. PCR for meq revealed the presence of 1062 bp long product in all DNA samples. Meq sequences of Polish strains have shown 68 bp insert in n 12 field strains. These inserts cause frame shift that alter aminoacid sequence of Meq (Fig.2). The comparison of DNA sequences of meq of Polish and reference strains was 78.9 percent. Meanwhile the conducted PCR for LTR of REV resulted on occurrence of products 0.8 kb long in 28 MDV strains. Moreover an additional 0.78 kb band was detected in two strains while 1.6 kb insert was also found in case of 2 other strains. Interestingly in 21 DNA samples a fine PCR products ranging from 0.8 kb to 2.0 kb were observed as a background.
PCR products for meq oncogene and LTR fragment were aligned with reference MD and REV sequences accessible in NCBI Genebank. In case of LTR sequences of REV retrieved from 10 recombinant Polish strains the similarity was 70.1 percent.
Total DNA was extracted directly from infected CEK cells using QIAamp Mini Kit (Qiagen) according to manufacturer´s requirements.
PCR was conducted for the amplification of meq oncogene and was described elsewhere [11]. PCR products were separated in 1.5% ethidium bromide stained agarose gels and were purified using Nucleospin Kit (Macherey-Nagel) PCR for LTR. The sequences of used primers specific for LTR-REV were described elsewhere (2). Reaction conditions were identical with the amplification of meq.
PCR products of meq from 24 MDV strains and 10 products for the LTR region were sequenced from forward and reversed primers on GS FLX/Titanium sequencer (Roche). The meq and LTR sequences were assembled and aligned using Genious ver. 5.1 [4].
Results. CPE was observed in all infected cell cultures starting from the 96h of 3rd passage. Infected cells formed focuses and syncytias. The results obtained by IFA have shown no influence of the observed insertion of the obtained results (Fig.1). DNA extracted from the infected cell cultures was used for the further study. PCR for meq revealed the presence of 1062 bp long product in all DNA samples. Meq sequences of Polish strains have shown 68 bp insert in n 12 field strains. These inserts cause frame shift that alter aminoacid sequence of Meq (Fig.2). The comparison of DNA sequences of meq of Polish and reference strains was 78.9 percent. Meanwhile the conducted PCR for LTR of REV resulted on occurrence of products 0.8 kb long in 28 MDV strains. Moreover an additional 0.78 kb band was detected in two strains while 1.6 kb insert was also found in case of 2 other strains. Interestingly in 21 DNA samples a fine PCR products ranging from 0.8 kb to 2.0 kb were observed as a background.
PCR products for meq oncogene and LTR fragment were aligned with reference MD and REV sequences accessible in NCBI Genebank. In case of LTR sequences of REV retrieved from 10 recombinant Polish strains the similarity was 70.1 percent.
Discussion. The presented study are concerned on molecular characteristics and changes of MDV field strains on the basis of molecular differences in Meq among Polish strains. The obtained results from sequencing of this gene in 24 strains revealed frame shift in 10 recently isolated field strains. The previous study have shown MDV may exist in coinfected chicken cells with other retroviruses [9, 10, 13]. Recombination between them was studied in chicken and duck embryo fibroblasts (CEF and DEF) [6, 7]. In this study recombinant MDV strains harboring LTR inserts were passaged 3 times in cell cultures without losing LTR fragment. This may suggest stable character of the insert. However an evidence on the role of LTR ion was given by Sun et al. [9] who studied pathogenicity of MDV-REV BAC clone GX101 in vivo. The performed IFA assay with FD7 anti-Meq antibodies revealed no influence of LTR insert on the antigenic features of Meq. However, sequencing of meq oncogene conducted in this study have shown a 68 bp insert among a group of 10 recently isolated field strains. This mutation had an influence on aminoacid sequence of Meq. Moreover among attenuated strains (CU-2, JM/102W or Rispens) 180 bp insert is associated with attenuation or at least lowered oncogenicity. Surprisingly, insert of Polish MDV strains had no lowering influence on its oncogenicity since these strains were isolated from chickens with severe MD symptoms and lesions. An another evidence is the origin of recombinant MDV strains that which were vaccinated birds. The 0.8 kb LTR insert into MDV genome was found in 28 strains and can be assigned as a most efficiently transcribed LTR region within SORF2 fragment of MDV that may influence transactivation of MDV genes [8].
Conclusion. The observed insertal mutation of meq gene of MDV with LTR region of REV may lead to its structural and functional changes. However these findings on the potential role and reasons of changing meq sequence need to be explained in the future.
Conclusion. The observed insertal mutation of meq gene of MDV with LTR region of REV may lead to its structural and functional changes. However these findings on the potential role and reasons of changing meq sequence need to be explained in the future.
References
1. Brown A.C., Baigent S.J., Smith L.P., Chattoo J.P., Petherbridge L.J., Hawes P., Allday M.J., Nair V.: Interaction of MEQ protein and C-terminal-binding protein is critical for induction of lymphomas by Marek´s disease virus. Proc. Natl. Acad. Sci. USA 103, 1687-1692, 2006.
2. I. Davidson, R. Borenshtain, H.J. Kung, R.L. Witter. Molecular indications for in vivo integration of the avian leukosis virus, subgroup J-long terminal repeat into the Marek´s disease virus in experimentally dually-infected chickens. Virus Genes 24, 173-180, 2002.
3. I. Davidson, H. Borenshtain. In vivo events of retroviral long terminal repeat integration into Marek´s disease virus in commercial poultry: detection of chimeric molecules as a marker, Avian Dis. 45, 102-121, 2001.
4. A.J. Drummond, B. Ashton, S. Buxton, M. Cheung, A. Cooper, J. Heled, M. Kearse, R. Moir, S. Stones-Havas S. Sturrock, T. Thierer, A. Wilson. Geneious v5.1
5. A. Hirano, T. Wong. Functional interaction between transcriptional elements in the long terminal repeat of reticuloendotheliosis virus: cooperative DNA binding of promoter- and enhancer-specific factors. Mol. Cell Biol. 8, 5232-5244,1999.
6. R. Isfort, D. Jones, R. Kost, R. Witter, H.J. Kung. Retrovirus insert into herpesvirus in vitro and in vivo. Proc. Natl. Acad. Sci. USA 89, 991-995, 1992.
7. D. Jones, R. Isfort, R. Witter, R. Kost, H.J. Kung. Retroviral inserts into a herpesvirus are clustered at the junctions of the short repeat and short unique sequences. Proc. Natl. Acad. Sci. USA 90, 3855-3859, 1993.
8. M. S. Parcells, A.S. Anderson, J.L Cantello, R.W. Morgan. Characterization of Marek´s disease virus insert and deletion mutants that lack US1 (ICP22 homolog), US10, and/or US2 and neighboring short-component open reading frames. J. Virol. 68, 8239-8253, 1994.
9. A. Sun, X. Xu, L. Petherbridge, Y. Zhao, V. Nair, Z. Cui. Functional evaluation of the role of reticuloendotheliosis virus long terminal repeat (LTR) integrated into the genome of a field strain of Marek´s disease virus. Virology 397, 270-276, 2010.
10. R.L. Witter, D. Li., D. Jones, L.F. Lee, H.J Kung. Retroviral insertal mutagenesis of a herpesvirus: a Marek´s disease virus mutant attenuated for oncogenicity but not for immunosuppression or in vivo replication. Avian Dis. 41, 407-421, 1997.
11. G. Wozniakowski, E. Samorek-Salamonowicz, W. Kozdrun. Sequence analysis of meq oncogene among Polish strains of Marek´s disease. Pol. J. Vet. Sci. 13, 263-267, 2010.
12. R.L. Witter, A.M. Fadly. Neoplastic diseases: Reticuloendotheliosis. In: Diseases of poultry. Ed By Y.M. Saif, A.M. Fadly, J.R. Glisson, L.R. McDougald, L.K. Nolan, D.E. Swayne (Blackwell Publishing, 2008, Ames, USA) pp. 515-529.
13. Z. Zhang, Z. Cui. Isolation of recombinant field strains of Marek´s disease virus integration with reticuloendotheliosis virus genome fragments. Sci. China C. Life Sci. 48, 81-88, 2005.
2. I. Davidson, R. Borenshtain, H.J. Kung, R.L. Witter. Molecular indications for in vivo integration of the avian leukosis virus, subgroup J-long terminal repeat into the Marek´s disease virus in experimentally dually-infected chickens. Virus Genes 24, 173-180, 2002.
3. I. Davidson, H. Borenshtain. In vivo events of retroviral long terminal repeat integration into Marek´s disease virus in commercial poultry: detection of chimeric molecules as a marker, Avian Dis. 45, 102-121, 2001.
4. A.J. Drummond, B. Ashton, S. Buxton, M. Cheung, A. Cooper, J. Heled, M. Kearse, R. Moir, S. Stones-Havas S. Sturrock, T. Thierer, A. Wilson. Geneious v5.1
5. A. Hirano, T. Wong. Functional interaction between transcriptional elements in the long terminal repeat of reticuloendotheliosis virus: cooperative DNA binding of promoter- and enhancer-specific factors. Mol. Cell Biol. 8, 5232-5244,1999.
6. R. Isfort, D. Jones, R. Kost, R. Witter, H.J. Kung. Retrovirus insert into herpesvirus in vitro and in vivo. Proc. Natl. Acad. Sci. USA 89, 991-995, 1992.
7. D. Jones, R. Isfort, R. Witter, R. Kost, H.J. Kung. Retroviral inserts into a herpesvirus are clustered at the junctions of the short repeat and short unique sequences. Proc. Natl. Acad. Sci. USA 90, 3855-3859, 1993.
8. M. S. Parcells, A.S. Anderson, J.L Cantello, R.W. Morgan. Characterization of Marek´s disease virus insert and deletion mutants that lack US1 (ICP22 homolog), US10, and/or US2 and neighboring short-component open reading frames. J. Virol. 68, 8239-8253, 1994.
9. A. Sun, X. Xu, L. Petherbridge, Y. Zhao, V. Nair, Z. Cui. Functional evaluation of the role of reticuloendotheliosis virus long terminal repeat (LTR) integrated into the genome of a field strain of Marek´s disease virus. Virology 397, 270-276, 2010.
10. R.L. Witter, D. Li., D. Jones, L.F. Lee, H.J Kung. Retroviral insertal mutagenesis of a herpesvirus: a Marek´s disease virus mutant attenuated for oncogenicity but not for immunosuppression or in vivo replication. Avian Dis. 41, 407-421, 1997.
11. G. Wozniakowski, E. Samorek-Salamonowicz, W. Kozdrun. Sequence analysis of meq oncogene among Polish strains of Marek´s disease. Pol. J. Vet. Sci. 13, 263-267, 2010.
12. R.L. Witter, A.M. Fadly. Neoplastic diseases: Reticuloendotheliosis. In: Diseases of poultry. Ed By Y.M. Saif, A.M. Fadly, J.R. Glisson, L.R. McDougald, L.K. Nolan, D.E. Swayne (Blackwell Publishing, 2008, Ames, USA) pp. 515-529.
13. Z. Zhang, Z. Cui. Isolation of recombinant field strains of Marek´s disease virus integration with reticuloendotheliosis virus genome fragments. Sci. China C. Life Sci. 48, 81-88, 2005.
 |  |
| Image 1 | Image 2 |
 |  |
| Image 3 | Image 4 |
Fig.1. Immunofluoroescence assay (IFA) with FD7 anti-Meq antibodies in chicken embryo kidney cells (CEK). Three recombinant strains are shown. 1-51/09, 2-81/09, 3-82/09, Neg - non-infected CEK SPF.
Fig.2. An alignment showing aminoacid sequence of Meq protein of Polish and reference MDV strain.
Content from the event:
Related topics:
Authors:
National Veterinary Research Institute (Poland)
Recommend
Comment
Share

Would you like to discuss another topic? Create a new post to engage with experts in the community.
Featured users in Poultry Industry

Nelson Ward
dsm-Firmenich
Manager, New Business Development at DSM Nutritional Products Inc.
United States
United States