How do Broiler Breeders and In-ovo Feeding Impact on Chick Quality?
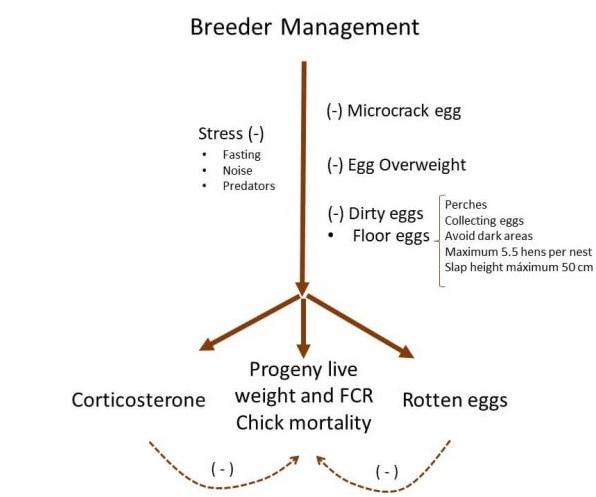
- Progeny live performance and chick mortality are affected by rotten eggs and corticosterone of broiler breeders. Thus, stress conditions elevate egg corticosterone, while egg management as microcrack, overweight, and dirty eggs influences the rotten eggs.
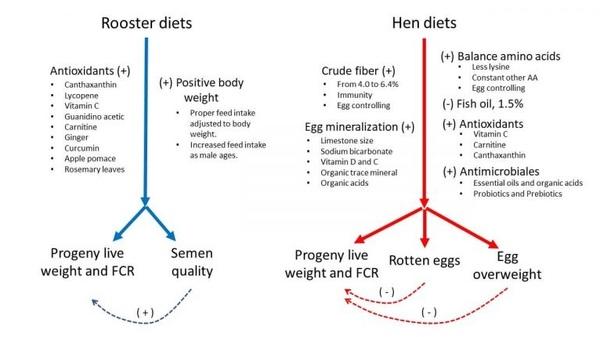
- Positive body weight gain and dietary antioxidants in roosters promote the semen quality, progeny live weight, and offspring feed conversion. Hen dietary macronutrients, minerals, vitamins, antioxidants, and antimicrobials yield better progeny performance by controlling the egg weight and rotten eggs, whereas fish oil reduces the chick quality.
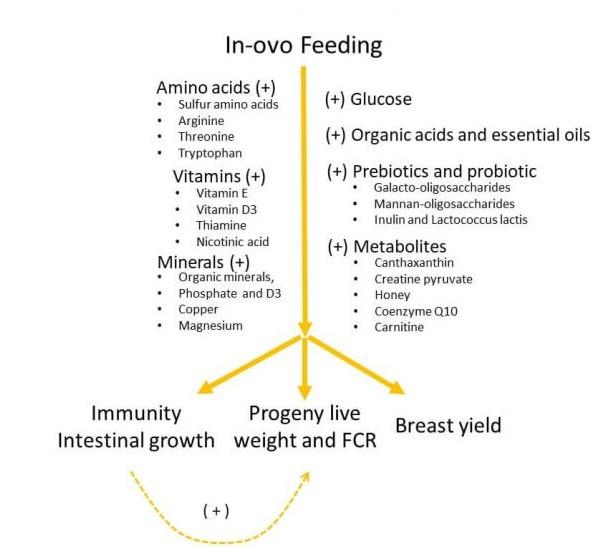
- In-ovo feeding of glucose, amino acids, minerals, vitamins, organic acids, essential oils, prebiotics, probiotics, and metabolites promote the broiler performance. On the other hand, enrichment of some dietary breeder vitamins and trace minerals might influence the egg content and chick quality.
Abdullah, S., I. H Leghari, A. A. Moriani, N. Rajput, J. A. Gandhai, and M. Nisa. 2018. Effect of in ovo supplementation of honey in fertile eggs on posthatch growth performance of broiler chickens. J. Anim. Plant Sci. 28:1584-1590.
Abe, E., H. Horikawa, T. Masumura, M. Sugahara, M. Kubota, and T. Suda. 1982. Disorders of cholecaiciferol metabolism in old egg-laying hens. J. Nutr. 112:436-446.
Akhlaghi, A., Y. Jafari Ahangari, B. Navidshad, Z. Ansari Pirsaraei, M. Zhandi, H. Deldar, M. R. Rezvani, M. Dadpasand, S. R. Hashemi, R. Poureslami, and E. D. Peebles. 2014a. Improvements in semen quality, sperm fatty acids, and reproductive performance in aged Cobb 500 breeder roosters fed diets containing dried ginger rhizomes (Zingiber officinale). Poult. Sci. 93:1236-1244.
Akhlaghi, A., Y. Jafari Ahangari, M. Zhandi, and E. D. Peebles. 2014b. Reproductive performance, semen quality, and fatty acid profile of spermatozoa in senescent broiler breeder roosters as enhanced by the long-term feeding of dried apple pomace. Anim. Reprod. Sci. 147:64-73.
Alzahrani, H. A., R. Alsabehi, L. Boukraa, F. Abdellah, Y. Bellik, and B. A. Bakhotmah. 2012. Antibacterial and antioxidant potency of floral honeys from different botanical and geographical origins. Molecules 17:10540-10549.
Arafat, A. R., H. A. Hassan, K. Y. Farroh, S. S. Elnesr, I. EL-wardany, and M. S. Bahnas. 2019. Effects of copper (sulfate, acetate and nano) in ovo injection on hatching traits and some physiological parameters of newly-hatched broiler chicks. J. Adv. Lab. Res. Biol. 10:65-72.
Araujo, I., M. B. Café, R. A. Noleto, J. Martins, C. J. Ulhoa, G. C. Guareshi, M. M. Reis, and N. S. Leandro. 2018. Effect of vitamin E in ovo feeding to broiler embryos on hatchability, chick quality, oxidative state, and performance. Poult. Sci. 98:3652-3661.
Araujo, L. F., C. S. S. Araujo, R. J. G. Pereira, L. C. Bittencourt, C. C., Silva, F. Cisneros, R. G. Hermes, Y. G. A. Sartore, and M. T. Dias. 2019. The dietary supplementation of canthaxanthin in combination with 25OHD3 results in reproductive, performance, and progeny quality gains in broiler breeders. Poult. Sci. 98:5801-5808.
Araujo, L. F., M. Bonato, R. Barbalho, C.S. D. S. Araujo, P. S., Zorzetto, C. A. Granghelli, R. J. G. Pereira, and A. j. T. Kawaoku. 2018. Evaluating hydrolyzed yeast in the diet of broiler breeder hens. J. Appl. Poult. Res. 27:65-70.
Bahakaim, A. S. A., and H. A. Abdel, S. M. H. Osman, A. S. Omar, N. Y. A. Malak, and N. A. Ramadan. 2014. Effect of using different levels and sources of zinc in layer’s diets on egg zinc enrichment. Egyptian Poultry Science Journal. 34:39-56.
Balloun, S. L., and R. E. Phillips. 1957. Interaction effects of vitamin B12 and pantothenic acid in breeder hen diets on hatchability, chick growth and livability. Poult. Sci. 36:929-934.
Barnett, D. M., B. L. Kumpula, R. L. Petryk, N. A. Robinson, R. A. Renema, and F. E. Robinson. 2004. Hatchability and early chick growth potential of broiler breeder eggs with hairline cracks. J. Appl. Poult. Res. 13:65-70.
Beck, C. N., C. D. McDaniel, K. GS Wamsley, and A.S. Kiess. 2019. The potential for inoculating Lactobacillus animalis and Enterococcus faecium alone or in combination using commercial in ovo technology without negatively impacting hatch and post-hatch performance. Poult. Sci. 98:7050-7062.
Bentley, O. G., and T. V. Hershberger. 1954. The Effect of Antibiotics on Hatchability of Hens’ Eggs and Progeny Growth Performance. Poult. Sci. 33:641-648.
Berwanger, E., S. L. Vieira, C. R. Angel, L. Kindlein, A. N. Mayer, M. A. Ebbing, and M. Lopes. 2018. Copper requirements of broiler breeder hens. Poult. Sci. 97:2785-2797.
Bhanja, S. K., A. B. Mandal, S. Majumdar, M. Mehra, and A. Goel. 2012. Effect of in ovo injection of vitamins on the chick weight and post-hatch growth performance in broiler chickens. Indian Journal of Poultry Science. 47:306-310.
Borghei-Rad, S. M., S. Zeinoaldini, M. Zhandi, H. Moravej, and M. Ansari. 2017. Feeding rosemary leaves powder ameliorates rooster age-related subfertility. Theriogenology 101:35-43.
Brewer, L. E., and H. M. Edwards Jr. 1972. Studies on the biotin requirement of broiler breeders. Poult. Sci. 51:619-624.
Cheled-Shoval, S. L., E. Amit-Romach, M. Barbakov, and Z. Uni. 2011. The effect of in ovo administration of mannan oligosaccharide on small intestine development during the pre-and posthatch periods in chickens. Poult. Sci. 90:2301-2310.
Chung, M. K., J. H. Choi, Y. K. Chung, and K. M. Chee. 2005. Effects of dietary vitamins C and E on egg shell quality of broiler breeder hens exposed to heat stress. Asian Australas. J. Anim. Sci. 18:545-551.
Ciacciariello, M., and N. C. Tyler. 2013. The effects of maternal dietary lysine intake on offspring performance to 21 days of age. J. Appl. Poultry Res. 22:238-244.
Coskun, I., A. Akkan, and G. Erener. 2018. Effects of in ovo injection of lysine and methionine into fertile broiler (parent stock) eggs on hatchability, growth performance, caecum microbiota, and ileum histomorphology. R. Bras. Zootec. 47:e20170220.
Dankowiakowska, A., J. Bogucka, A. Sobolewska, S. Tavaniello, G. Maiorano, and M. Bednarczyk. 2019. Effects of in ovo injection of prebiotics and synbiotics on the productive performance and microstructural features of the superficial pectoral muscle in broiler chickens. Poult. Sci. 98:5157-5165.
Dayangac, A., M. Bahsi, A. Ozkaya, and O. Yilmaz. 2011. Linalool improve biochemical damage and fatty acids composition of testes on fasting male rats. J. Anim. Vet. Adv. 10:1232-1238.
Denton, C. A., W. L. Kellogg, J. R. Sizemore, and R. J. Lillie. 1954. Effect of injecting and feeding vitamin B12 to hens on content of the vitamin in the egg and blood: One figure. J. Nutr. 54:571-577.
Dooley, M. R. 2011. Evaluation of L-carnitine in ovo injection followed by L-carnitine feed supplementation on broiler hatching and growing characteristics. PhD Diss. Mississippi State Univ.
Ekmay, R. D., M. De Beer, R. W. Rosebrough, M. P. Richards, J. P. McMurtry, and C. N. Coon. 2010. The role of feeding regimens in regulating metabolism of sexually mature broiler breeders. Poult. Sci. 89:1171-1181.
Elnesr, S. S., H. A. M. Elwan, Q. Q. Xu, C. Xie, X. Y. Dong, and X. T. Zou. 2019. Effects of in ovo injection of sulfur-containing amino acids on heat shock protein 70, corticosterone hormone, antioxidant indices, and lipid profile of newly hatched broiler chicks exposed to heat stress during incubation. Poult. Sci. 98:2290-2298.
Elwan, H. A., S. S. Elnesr, Q. Xu, C. Xie, X. Dong, and X. Zou. 2019. Effects of in ovo methionine-cysteine injection on embryonic development, antioxidant status, IGF-I and TLR4 gene expression, and jejunum histomorphometry in newly hatched broiler chicks exposed to heat stress during Incubation. Animals. 9:25. doi:10.3390/ani9010025
Enting, H., T. A. M. Kruip, M. W. A. Verstegen, and P. J. Van der Aar. 2007. The effect of low-density diets on broiler breeder performance during the laying period and on embryonic development of their offspring. Poult. Sci. 86:850-856.
Fatemi, S. A., K. E. C. Elliott, A. Bello, O. A. Durojaye, H. J. Zhang, and E. D. Peebles. 2019. The effects of in ovo injected vitamin D3 sources on the eggshell temperature and early posthatch performance of Ross 708 broilers. Poult. Sci. https://doi.org/10.1016/j.psj.2019.10.055
Frank, F.R., and R. E. Burger. 1965. The effect of carbon dioxide inhalation and sodium bicarbonate ingestion on egg shell deposition. Poult. Sci. 44:1604-1606.
Fuller, H. L., R. C. Field, R. Roncalli-Amici, W. S. Dunahoo, and H. M. Edwards Jr. 1961. The vitamin B6 requirement of breeder hens. Poult. Sci. 40:249-253.
Galobart, J., A. C. Barroeta, M. D. Baucells, and F. Guardiola. 2001. Lipid oxidation in fresh and spray-dried eggs enriched with ω3 and ω6 polyunsaturated fatty acids during storage as affected by dietary vitamin E and canthaxanthin supplementation. Poult. Sci. 80:327-337.
Gholami, J., A. A. Qotbi, A. Seidavi, A., Meluzzi, S. Tavaniello, and G. Maiorano. 2015. Effects of in ovo administration of betaine and choline on hatchability results, growth and carcass characteristics and immune response of broiler chickens. Italian Journal of Animal Science. 14:187-192.
Gholami, M., A. Seidavi, C. J. O’Shea, Y. Akter, M. Dadashbeiki, and B. Bahar. 2017. Feeding regimen of breeder broiler hen influences growth performance of the broiler chickens. Livest. Sci. 203:132-135.
Glodek, K., M. W. Lis, B. Płytycz, A. Mazur, and J. W. Niedziółka. 2010. Effect of high dose of riboflavin injected in ovo on hatchability and gain of broiler chicken. Anim. Biol. 12:269-275
House, J. D., K. Braun, D. M. Ballance, C. P. O’connor, and W. Guenter. 2002. The enrichment of eggs with folic acid through supplementation of the laying hen diet. Poult. Sci. 81:1332-1337.
Ismail, F. S. A., K. Sherif, Y. S. Rizk, and M. E. Hassan. 2019. Effect of spirulina and canthaxanthin injection into hatching eggs on hatchability traits and subsequent growth performance of chicks. Journal of Animal and Poultry Production. 10:197-202.
Jiang, Y. H., R. B. McGeachin, and C. A. Bailey. 1994. α-Tocopherol, β-carotene, and retinol enrichment of chicken eggs. Poult. Sci. 73:1137-1143.
Kadam MM, Bhanja SK, Mandal AB, Thakur R, Vasan P, Bhattacharyya A, and J. S. Tyagi. 2008. Effect of in ovo threonine supplementation on early growth, immunological responses and digestive enzyme activities in broiler chickens. Br. Poult. Sci. 49:736-741.
Kalantar, M., S. M. Hosseini, M. R. Hosseini, M. H. Kalantar, and L. G. Yang. 2019. Effects of in ovo injection of coenzyme Q10 on hatchability, subsequent performance, and immunity of broiler chickens. BioMed Res. Inter. https://doi.org/10.1155/2019/7167525
Kazemizadeh, A., A. Z. Shahneh, S. Zeinoaldini, A. R. Yousefi, H. M. Yeganeh, Z. A. Pirsaraei, and A. Akhlaghi. 2019. Effects of dietary Curcumin supplementation on seminal quality indices and fertility rate in broiler breeder roosters. Br. Poult. Sci. DOI: 10.1080/00071668.2019.1571165
Keke, A. and I. Cinkmanis. 2019. Determination of organic acids in honey samples from latvian market by high-performance liquid chromatography. Research for Rural Development. 1: DOI: 10.22616/rrd.25.2019.034
Khan, R. U., Z. U. Rahman, I. Javed, and F. Muhammad. 2013 Effect of vitamins, probiotics and protein level on semen traits and seminal plasma biochemical parameters of post-moult male broiler breeders. Br. Poult. Sci. 54:120-129.
Khong, C., S. Sen, S. Lee, Y. Choi, K. Y. Kim, S. Ingale, and I. K. Kwon. 2014. Effect of sodium butyrate supplementation on performance, egg quality and bacterial load in the excreta of laying hens. J. Anim. Res. 4:141-153.
Kidd, M. T., L. Araujo, C. Araujo, C. D. McDaniel, and D. McIntyre. 2013. A study assessing hen and progeny performance through dam diet fortification with a Saccharomyces cerevisiae fermentation product. J. Appl. Poult. Res. 22:872-877.
Koppenol, A., E. Delezie, Y. Wang, L. Franssens, E. Willems, B. Ampe, B, J. Buyse, and N. Everaert. 2015. Effects of maternal dietary EPA and DHA supplementation and breeder age on embryonic and post-hatch performance of broiler offspring. J. Anim. Physiol. A. Anim. Nutr. 99:36-47.
Li, L. L., N. N. Zhang, Y. J. Gong, M. Y. Zhou, H. Q. Zhan, and X. T. Zou. 2018. Effects of dietary Mn-methionine supplementation on the egg quality of laying hens. Poult. Sci. 97:247-254.
Lu, J., L. Qu, M. M. Shen, X. G. Wang, J. Guo, Y. P. Hu, T. C. Dou, and K. H. Wang. 2019. Effects of high-dose selenium-enriched yeast on laying performance, egg quality, clinical blood parameters, organ development, and selenium deposition in laying hens. Poult. Sci. 98:2522-2530.
Maiorano, G., A. Sobolewska, D. Cianciullo, K. Walasik, G. Elminowska-Wenda, A. Sławińska, S. Tavaniello, J. Zylińska, J. Bardowski, and M. Bednarczyk. 2012. Influence of in ovo prebiotic and synbiotic administration on meat quality of broiler chickens. Poult. Sci. 91:2963-2969.
Manangi, M. K., M. Vazques-Añon, J. D. Richards, S. Carter, and C. D. Knight. 2015. The impact of feeding supplemental chelated trace minerals on shell quality, tibia breaking strength, and immune response in laying hens. J. Appl. Poult. Res. 00:1-11.
Mangiagalli, M. G., P. A. Martino, T. Smajlovic, L. Guidobono Cavalchini, and S. P. Marelli. 2010. Effect of lycopene on semen quality, fertility and native immunity of broiler breeder. Br. Poult. Sci. 51:152-157.
Mato, I. S., J. F. Huidobro, J. Jesu, J. Simal-Lozano, and A. M. T. Sancho. 2003. Significance of Nonaromatic organic acids in honey. Journal of Food Protection. 66:2371-2376.
Mattila, P., J. Valaja, L. Rossow, E. Venäläinen, and T. Tupasela. 2004. Effect of vitamin D2-and D3-enriched diets on egg vitamin D content, production, and bird condition during an entire production period. Poult. Sci. 83:433-440.
Mejia, L., C. D. McDaniel, M. T. Kidd, K. Lopez, and A. Corzo. 2013. Evaluation of carryover effects of dietary lysine intake by Cobb 500 broiler breeder hens. Poult. Sci. 92:709-718.
Memon, S. S., A. A. Kamboh, I. H. Leghari, and R. A. Leghari. 2019. Effect of in ovo and post-hatch administration of honey on the immunity and intestinal microflora of growing chickens. J. Anim, Feed Sci. 28:346-353.
Momeneh, T., and M. Torki. 2018. Effects of in ovo injection of vitamins B 6 and B 12 in fertile eggs subjected to ethanol stress on hatching traits, performance and visceral organs of broiler chicks reared under cold stress condition. Iran. J. Appl. Anim. Sci. 8:491-498.
Moreira-Filho, A. L. B., P. R. Ferket, R. D. Malheiros, C. J. B. Oliveira, P. C. Aristimunha, D. E. Wilsmann, and P. E. N. Givisiez. 2018. Enrichment of the amnion with threonine in chicken embryos affects the small intestine development, ileal gene expression and performance of broilers between 1 and 21 days of age. Poult. Sci. 98:1363-1370.
Naber, E. C. 1979. The effect of nutrition on the composition of eggs. Poult. Sci. 58:518-528.
Naber, E. C. and M. W. Squires. 1993. Vitamin profiles of eggs as indicators of nutritional status in the laying hen: diet to egg transfer and commercial flock survey. Poult. Sci. 72:1046-1053.
Nayak, N., R. A. Rajini, J. J. Kirubaharan, S. Ezhilvalavan, and A. R. Sahu. 2018. Effect of In Ovo Feeding of Tryptophan on Post-Hatch Production Performance and Immune Response in Commercial Broilers. Anim. Nutr. Feed Techn. 18:355-366.
Opaliński, S., B. Dolińska, M. Korczyński, K. Chojnacka, Z. Dobrzański, and F. Ryszka. 2012. Effect of iodine-enriched yeast supplementation of diet on performance of laying hens, egg traits, and egg iodine content. Poult. Sci. 91:1627-1632.
Owen, A. L. 2017. Using a direct-fed microbial in broiler breeders to reduce broiler progeny lameness. M.S. Diss. Univ. Georgia. Athens.
Park, S. W., H. Namkung, H. J. Ahn, and I. K. Paik. 2005. Enrichment of vitamins D3, K and iron in eggs of laying hens. Asian-Aust. J. Anim. Sci. 18:226-229.
Parnian, A., B. Navidshad, F. Mirzaei, R. Behmaram, and H. Deldar, H. 2019. Effect of in ovo injection of nicotonic acid, pantothenic acid or folic acid on immune system and growth of broiler chickens. Iran J. Vet. Med. 13:411-420.
Polin, D., W. H. Ott, E. R. Wynosky, and C. C. Porter. 1963. Estimation of thiamine requirement for optimum hatchability from the relationship between dietary and yolk levels of the vitamin. Poult. Sci. 42:925-928.
Pruszynska-Oszmalek, E., P. A. Kolodziejski, K. Stadnicka, M. Sassek, D. Chalupka, B. Kuston, L. Nogowski, M. P., Maiorano, G., Jankowski, J. and M. Bednarczyk. 2015. In ovo injection of prebiotics and synbiotics affects the digestive potency of the pancreas in growing chickens. Poult. Sci. 94:1909-1916.
Ramadan, N. A., A. S. Omar, A. S. A. Bahakaim, and S. M. Osman. 2010. Effect of using different levels of iron with zinc and copper in layer’s diet on egg iron enrichment. Int. J. Poult. Sci. 9:842-850.
Ringrose, R. C., A. G. Manoukas, R. Hinkson, and A. E. Teeri. 1965. The niacin requirement of the hen. Poult. Sci. 44:1053-1065.
Romero-Sanchez, H. 2005. Effect of male broiler breeder feeding programs on growth, reproductive performance, and broiler progeny. PhD Diss. North Carolina State University, Raleigh.
Rosa, A. P., A. Scher, J. O. B. Sorbara, L. S. Boemo, J. Forgiarini, and A. Londero. 2012. Effects of canthaxanthin on the productive and reproductive performance of broiler breeders. Poult. Sci. 91:660-666.
Saino, N., M. Romano, R. P. Ferrari, R. Martinelli, and A. P. Moller. 2005. Stressed mothers lay eggs with high corticosterone levels which produce low quality offspring. J. Exp. Zool. A. Comp. Exp. Biol. 303:998-1006.
Salmanzadeh, M., Y. Ebrahimnezhad, H. A. Shahryar, and R. Beheshti. 2012. The effects of in ovo injection of glucose and magnesium in broiler breeder eggs on hatching traits, performance, carcass characteristics and blood parameters of broiler chickens. Arch. Geflugelkunde. 76:277-84.
Sengor, E., M. Yardimci, S. Cetingul, L. Bayram, H. Sahin, and I. Dogan. 2007. Effects of short chain fatty acid (SCFA) supplementation on performance and egg characteristics of old breeder hens. S. Afr. J. Anim. Sci. 37:158-163.
Shafey, T. M., A. H. Mahmoud, A. A. Alsobayel, and M. A. Abouheif. 2014. Effects of in ovo administration of amino acids on hatchability and performance of meat chickens. South Afr. J. Anim. Sci. 44:123-130.
Scheideler, S. E. 1998. Eggshell calcium effects on egg quality and Ca digestibility in first-or third-cycle laying hens. J. Appl. Poult. Res. 7:69-74.
Slawinska, A., M. Zampiga, F. Sirri, A. Meluzzi, M. Bertocchi, S. Tavaniello, and G. Maiorano. 2020. Impact of galactooligosaccharides delivered in ovo on mitigating negative effects of heat stress on performance and welfare of broilers. Poult. Sci. 99:407-415.
Tabler, G. T., I. L. Berry, and A. M. Mendenhall. 2004. Mortality Patterns Associated with Commercial Broiler Production. In the Poultry Site. 5m Editor. Dec, 13.
Tapeh, R. S., M. Zhandi, M. Zaghari, and A. Akhlaghi. 2017. Effects of guanidinoacetic acid diet supplementation on semen quality and fertility of broiler breeder roosters. Theriogenology. 89:178-182.
Tavaniello, S., R. Mucci, K. Stadnicka, O. Acaye, M. Bednarczyk, and G. Maiorano. 2019. Effect of in ovo administration of different synbiotics on carcass and meat quality traits in broiler chickens. Poult. Sci. 98:464-472.
Toghyani, M., S. Tahmasebi, M. Modaresi, and S. S. Ale Saheb Fosoul. 2019. Effect of arginine and threonine in ovo supplementation on immune responses and some serum biochemical attributes in broiler chickens. Ital. J. Anim. Sci. 18:342-349.
Toosi, S., M. Chamani, M. Shivazad, A. A. Sadeghi, and S. N. Mousavi. 2016. Effects of in ovo injection and inclusion a blend of essential oils and organic acids in high NSPS diets of broiler breeders on performance of them and their offspring. J. Poult. Sci. 53:192-200.
Triques, G. E., A. B. D. Cristo, M. Canevese, P. F. D. S. Marques, A. M. Burin Junior, and J. I. M. Fernandes. 2019. Effect of antioxidant supplementation in diets of roosters during the post-peak phase on reproduction and production characteristics of offspring. Cienc. Anim. Bras. 20:e-43072.
Tsang, C. P. W. 1992. Research note: calcitriol reduces egg breakage. Poult. Sci. 71:215-217.
Ulmer-Franco, A. M., G. M. Fasenko, and E. E. O’Dea Christopher. 2010. Hatching egg characteristics, chick quality, and broiler performance at 2 breeder flock ages and from 3 egg weights. Poult. Sci. 89:2735-2742.
Valle, R. 2008. Estimulando la postura de huevos en nido. Boletín de Servicio. Arbor Acres. May.
Van den Brand, H., M. P. Sosef, A. Lourens, and J. van Harn. 2016. Effects of floor eggs on hatchability and later life performance in broiler chickens. Poult. Sci. 95:1025-1032.
Xie, C., H. A. M. Elwan, S. S. Elnesr, X. Y. Dong, and X. T. Zou. 2019. Effect of iron glycine chelate supplementation on egg quality and egg iron enrichment in laying hens. Poult. Sci. 98:7101-7109
Yair, R., R. Shahar, and Z. Uni. 2015. In ovo feeding with minerals and vitamin D3 improves bone properties in hatchlings and mature broilers. Poult. Sci. 94:2695-2707.
Yoho D. E., J. R. Moyle, A. D. Swaffar, and R. K. Bramwell. 2008. Effect of incubating poor quality broiler breeder hatching eggs on overall hatchability and hatch of fertile. Poult. Sci. 87 (Suppl.1):148.
Youssef, A. W., E. F. El-Daly, N. A. A. El-Azeem, and M. M. El-Monairy. 2013. Effect of sodium formate on laying hen performance, gastrointestinal tract pH and some blood components under heat stress conditions. Asian J. Poult. Sci. 7:17-26.
Yu, L. L., T. Gao, M. M. Zhao, P. A. Lv, L. Zhang, J. L. Li, Y. Jiang, F. Gao, and G. H. Zhou. 2018. Effects of in ovo feeding of L-arginine on breast muscle growth and protein deposition in post-hatch broilers. Animal. 12:2256-2263.
Zhang, H., K. E. C. Elliott, O. A. Durojaye, S. A. Fatemi, M. W. Schilling, and E. D. Peebles. 2019. Effects of in ovo injection of L-ascorbic acid on growth performance, carcass composition, plasma antioxidant capacity, and meat quality in broiler chickens. Poult. Sci. 98:3617-3625.
Zhao, M. M., T. Gao, L. Zhang, J. L. Li, P. A. Lv, L. L. Yu, F. Gao, and G. H. Zhou. 2017. Effects of in ovo feeding of creatine pyruvate on the hatchability, growth performance and energy status in embryos and broiler chickens. Animal. 11:1689-1697.









_1.jpg&w=3840&q=75)