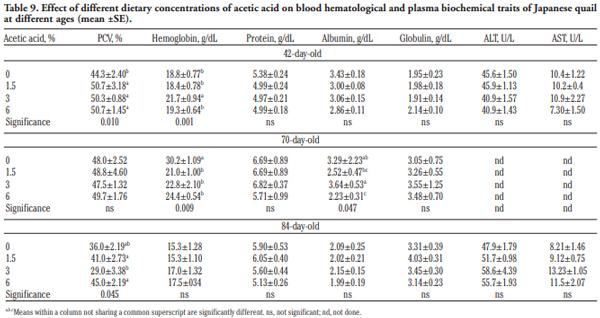
Growing and laying performance of Japanese quail fed diet supplemented with different concentrations of acetic acid
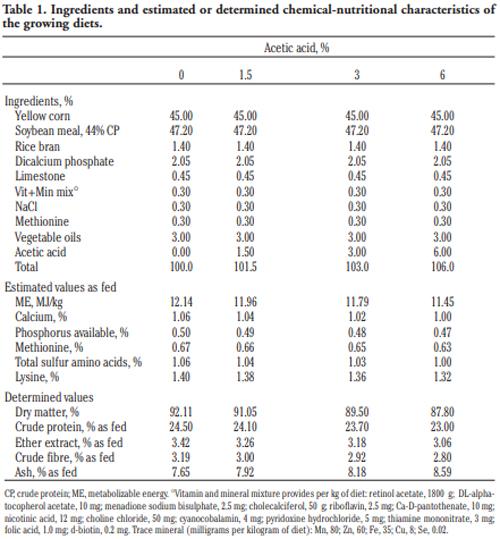
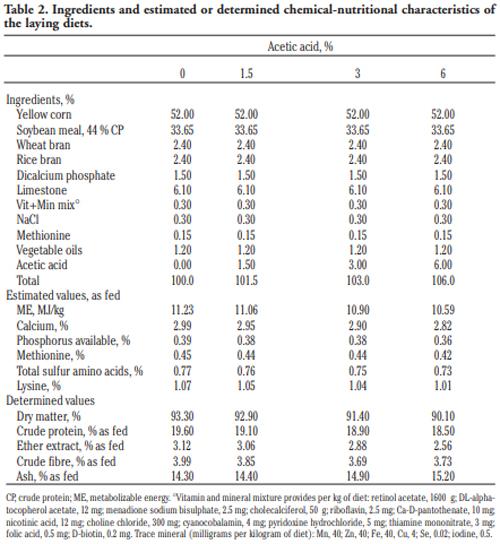
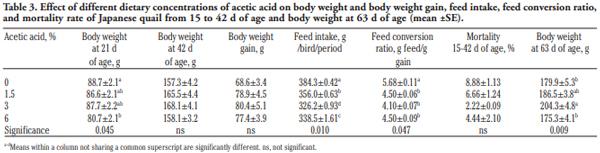
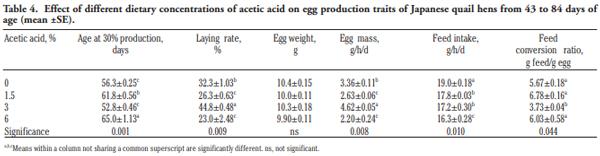
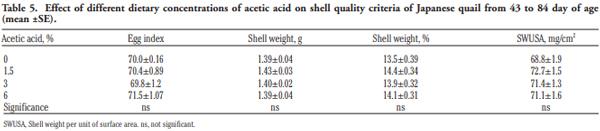
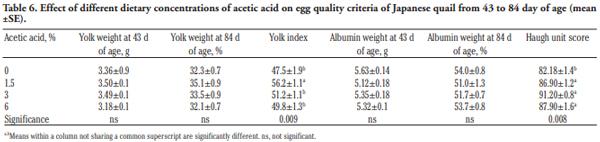
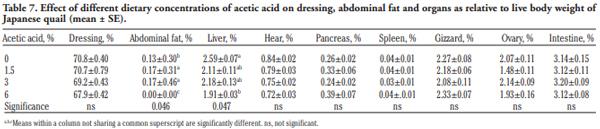
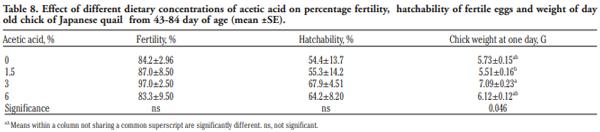
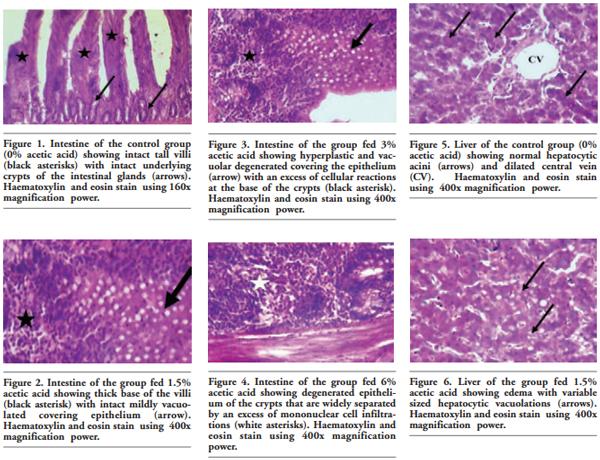
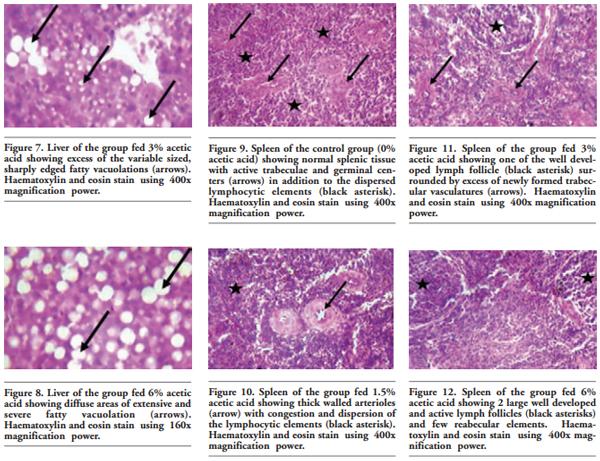
In order to evaluate the effect of acetic acid on growing and laying performance of Japanese quail (JQ), 180 15-day-old JQ were divided into 4 groups. During the growing (15- 42 days of age) and laying (43-84 days of age) periods, the groups fed the same basal diets supplemented with 0, 1.5, 3 and 6% of acetic acid. Each diet was fed to five replicates of 9 JQ (3 males:6 females) during the growing period. During the laying period, 128 birds were housed in 32 cages (4 birds per cage, 1 male and 3 females, 8 replicates per treatment). Birds were housed in wire cages (46L×43W× 20H cm) in an open room. Acetic acid supplementation at 3% in the diets significantly increased the growth and laying rate and the Haugh unit score. The liver percentage significantly decreased with acetic acid at 6%. Acetic acid at 3% significantly increased hemoglobin concentrations at 6 weeks of age and increased weight of day old chicks hatched. Acetic acid affected the immune system as manifested by an excess of cellular reactions in the intestine as well as lymphoid hyperplasia in the spleen tissue. Degenerative changes in the covering epithelium of the intestinal villi were noted at the 6% concentration of acetic acid. Hepatocyte vacuolation and fatty changes were also observed at this concentration of treatment. In conclusion, 3% acetic acid may be used as a feed supplement for JQ during the growing and laying period to improve the productive performance.
Abdel-Fattah, S.A., El-Sanhoury, M.H., ElMednay, N.M., Abdel-Azeem, F.F., 2008. Thyroid activity, some blood constituents, organs morphology and performance of broiler chicks fed supplemental organic acids. Int. J. Poultry Sci. 7:215-222.
Armstrong, W.D., Carr, C.W., 1964. Physiological chemistry laboratory directions, 3rd ed., Burges Publishing Co., Minneapolis, MS, USA.
Attia, Y.A., Böhmer, B.M., Roth-Maier, D.A., 2006. Responses of broiler chicks raised under constant relatively high ambient temperature to enzymes, amino acid supplementations, or diet density. Arch. Geflugelkd. 70:80-91.
Attia, Y.A., Burke, W.H., Yamani, K.A., 1994. Response of broiler breeder hens to forced molting by hormonal and dietary manipulations. Poultry Sci. 73:245-258.
Attia, Y.A., Burke, W.H., Yamani, K.A., Jensen, L.S., 1995. Daily energy allotments and performance of broiler breeders. 2. Females. Poultry Sci. 74:261-270.
Attia, Y.A., Ellakany, H.F., Abd El-Hamid, A.E., Bovera, F., Ghazaly, S.A., 2012. Control of Salmonella enteritidis infection in male layer chickens by acetic acid and/or prebiotics, probiotics and antibiotics. Arch. Geflugelkd. 76:239-245.
Bancroft, J.D., Marilyn, G., 2002. Theory and practice of histological techniques, 5th ed. Churchill-Livingstone Publ., London, UK.
Bovera, F., Moniello, G., De Riu, N., Di Meo, C., Pinna, W., Nizza, A., 2007. Effect of diet on the metabolic profile of ostriches (Struthio camelus var. domesticus). Trop. Anim. Health Prod. 39:265-270.
Çelik, K., Ersoy, E., Uzatici, A., Erturk, M., 2003. The using of organic acids in California turkey chicks and its effects on performance before pasturing. Int. J. Poultry Sci. 2:446-448.
Çelik, L., Bozkurt Kiraz, A., Bozkurt, Z., Kutlu, H.R., 2008. Effects of dietary prebiotic on performance, egg quality and some plasma parameters of laying hens exposed to heat stress. pp 28-35 in Proc. 23rd World Poultry Congr., Brisbane, Australia.
Christian, L., Nizamettin, S., Hasan, A., Aylin, A., 2004. Acidifier, a modern alternative for anti-biotic free feeding in livestock production, with special focus on broiler production. Vet. Zootec. 27:564 -568.
Coles, E.H., 1974. Veterinary clinical pathology, 2nd ed. W.B. Saunders Co. Publ., Philadelphia, PA, USA.
Denli, M., Ferda, O., Kemal, C., 2003. Effect of dietary probiotic, organic acid and antibiotic supplementation to diets on broiler performance and carcass yield. Pak. J. Nutr. 2:89-91.
Doumas, B.T., Watson, W., Biggs, H.G., 1977. Albumin standards and the measurement of serum albumin with bromocresol green. Clin. Chem. Acta 31:87-96.
Eilers, R.I., 1967. Notification of final adoption of an international method and standard solution for hemoglobin specification for preparation of standard solutions. Am. J. Clin. Pathol. 47:212-214.
El-Afifi, S.F., 2003. Comparative studies on garlic bulb, virginiamycin, copper sulfate and citric acid as growth promoters in broiler chick’ diets. J. Agric. Sci. 28:3423-3432.
El-Afifi, S.F., EL-Medney, N.M., Attia, M., 2001. Effect of citric acid supplementation in broiler diets on performance and intestinal microflora. Egypt. Poultry Sci. J. 21:491- 505.
El-Deek, A.A., Al-Harthi, M.A., Attia, Y.A., 2011. Effect of different dietary levels of dried eggs by-product without or with shell and/or premix on the performance of laying strain chicks from 2 to 8 wk of age. Arch. Geflugelkd. 75:169-178.
El-Hommosany, Y.M., 2008. Study of the physiological changes in blood chemistry, humoral immune response and performance of quail chicks fed supplemental chromium. Int. J. Poultry Sci. 7:40-44.
Farran, M.T., Barbour, G.W., Uwayjan, M.G., Ashkarian, V.M., 2001a. Metabolizable energy values and amino acid availability vetch, Vicia sativa and ervil, Vicia ervilia seeds soaked in water acetic acid. Poultry Sci. 80:931-936.
Farran, M.T., Dakessian, P.B., Darwish, A.H., Uwayjan, M.G., Dbouk, H.K., Sleiman, F.T., Ashkarian, V.M., 2001b. Performance of broilers and production and egg quality parameters of laying hens fed 60% raw or treated common vetch, Vicia sativa seeds. Poultry Sci. 80:203-208.
Garcia, V., Gregori, C., Hernandez, F., Megias, M.D., Madrid, J., 2007. Effect of formic acid and plant extracts on growth, nutrient digestibility, intestine mucosa morphology, and meat yield of broilers. J. Appl. Poultry Res. 16:555-562.
Gunal, M., Yayli, G., Kaya, O., Karahan, N., Sulak, O., 2006.The effects of antibiotic growth promoter, probiotic or organic acid supplementation on performance, intestinal microflora and tissue of broilers. Int. J. Poultry Sci. 5:149-155.
Hernandez, F., Garcia, V., Madrid, J., Orengo, J., Catala, P., Megias, M.D., 2006. Effect of formic acid on performance, digestibility, intestinal histomorphlogy and plasma metabolite levels of chickens. Brit. Poultry Sci. 47:50-56.
Hume, M.E., Corrier, D.E., Ambrus, S., Hinton, A., Eloach, J.R., 1993. Effectiveness of dietary propionic acid in controlling Salmonella typhimurium colonization in broiler chicks. Avian Dis. 37:1051-1056.
Incharoen, T., Yamauchi, K., Erikawa, T., Gotoh, H., 2010. Histology of intestinal villi and epithelial cells in chickens fed lowcrude protein or low-crude fat diets. Ital J. Anim. Sci. 9:e82.
Kim, C.R., Marshall, D.L., 2000. Quality evaluation of refrigerated chicken wings treated with organic acids. J. Food Qual. 23:327- 335.
Kirchgessner, M., Roth, F.X., 1988. Ergotrope Effekte durch organische Säuren in der Ferkelaufzucht und Schweinemast. Übersichten zur Tierernährung 16:93-108.
Kirchgessner, M., Roth, F.X., Steinruck, U., 1991. Wirkung von fumarsaure bei anderung der proteinqualitat und des proteingenaltes imfutter auf die mastlestug von broilern. Arch. Geflugelkd. 55:224-232.
Koley, S., Ghosh, H., Paul, S.K., Halder, G., Samanta, G., 2008. Organic aids as a substitute of antibiotic growth promoters in Japanese quail broiler chicken and ducks. pp 102-107 in Proc. 23rd World Poultry Congr., Brisbane, Australia.
Moniello, G., Bovera F., Solinas I.L., Piccolo, G., Pinna, W., Nizza, A., 2005. Effect of age and blood collection site on the metabolic profile of ostriches. S. Afr. J. Anim. Sci. 35: 268-272.
National Research Council, 1994. Nutrient requirement of poultry. 9th rev. ed. National Academy Press, Washington, DC, USA.
Paul, K.S., Gobinda, H., Manas, K., Mondal Gautam, S., 2007. Effect of organic acid salt on the performance and gut health of broiler chicken. J. Poultry Sci. 44:389-395.
Reitman, S., Frankel, S., 1957. Calorimetric method for the determination of blood aminotransferase enzymatic activities. Am. J. Clin. Pathol. 28:56-63.
SAS, 2000. SAS user’s Guide, Statistic. SAS Inst. Inc., Cary, NC, USA.
Scholtz, N., Halle, I., Flachowsky, G., Sauerwein, H., 2009. Serum chemistry reference values in adult Japanese quail (Coturnix coturnix japonica) including sex-related differences. Poultry Sci. 88: 1186-1190.
Thompson, J.L., Hinton, M., 1997. Antibacterial activity of formic and propionic acids in the diet of hens on salmonellas in the crop. Poultry Sci. 38:59-65.
Vogt, H., Matthes, S., Harnisch, S., 1981. Der Einfluss organischer Säuren auf die Leistungen von Broilern und Legehennen. Arch. Geflugelkd. 45:221-232.
Yousefi, M., Kalantar, M., Karkoodi, K., Farivar, F., 2008. Application of dietary probiotics and organic acids on performance of broiler chickens. pp 134-139 in Proc. 23rd World Poultry Congr., Brisbane, Australia.
Zyla, K., Koreleski, J., Swiatkiewicz, S., Wikiera, A., Kujawski, M., Piironen, J., Ledoux, D.R., 2002. Effect of phosphorolytic and cell wall-degrading enzymes on the performance of growing broilers fed wheat-based diet containing different calcium levels. Poultry Sci. 79:66-76.










.jpg&w=3840&q=75)