INTRODUCTION
Coccidiosis presently proves to be a major and pressing protozoan disease in the poultry industry worldwide (Dalloul and Lillehoj, 2006). Coccidiosis is caused by a protozoan parasite from the genus Eimeria. The life cycle of coccidial parasites includes asexual and sexual replication stages and begins when a bird ingests sporulated oocysts from the environment, as described by Conway and McKenzie (2007). After ingestion, four sporocysts contained in a single sporulated oocyst release two sporozoites. The release of the sporozoites is caused by digestive activity within the chicken. Released sporozoites will then “invade epithelial cells in a specific zone of the intestine or ceca;” which is dependent on the Eimeria species (Chapman, 2003). Within the cell, sporozoites become trophozoites and feed for twelve to forty-eight hours to grow and eventually asexually divide via schizogony, or merogony; this stage is known as a schizont or meront. Within the parasite, the merozoite stages form and are released after the schizont matures and ruptures, which takes three days. This first generation of merozoites will invade more epithelial cells and repeat the multiplication process. The second generation of merozoites may induce a third schizogonous cycle; this too is dependent on the Eimeria species. Both male (microgametocytes) and female (macrogametocytes) gametocytes will form. Macrogametocytes will grow into macrogametes. Microgametocytes will mature, rupture, and release biflagellate microgametes that fertilize the female macrogametes. Following fertilization, a “thickened wall forms around the macrogamete, forming a zygote” (Conway and McKenzie, 2007; McDougald and Fitz-Coy, 2013). At the conclusion of this cycle, a new oocyst is formed and will pass through the bird’s droppings after rupturing its host cell (Tewari and Maharana, 2011).
Eimeria spp. oocysts, from a single or several simultaneous infections, are excreted in feces over a period of several days. Oocysts shedding starts low, reaches a plateau, and then decreases until the disease runs its course (Clarke, 1979; Williams, 1973). Interestingly, several investigators have reported that oocyst counts differ between morning and evening sampling collections (Hudman et al., 2000; Brown et al., 2001). This variability has been recognized for several years but has largely been overlooked (Misof, 2004). Recently, however, it has been shown that, the day post-inoculation and the time of day at which samples are collected can have a significant effect on the reliability and validity of the data (Brawner and Hill, 1999). Hence, the purpose of the present study was to evaluate the influence of oocyst shedding variation and day of sampling on experimental challenge with Eimeria maxima and Eimeria acervulina in broiler chickens.
MATERIALS AND METHODS
Challenge strains
Oocysts of Eimeria maxima M6 (EM) and wild-type E. acervulina (EA) were provided by Dr. John. R. Barta, University of Guelph, Canada. The methods for detecting and recovering oocysts from infected chickens, oocyst sporulation, and the preparation of infective doses, were conducted as described previously (Haug et al., 2006). A dose-titration study was performed to determine the EM/EA coccidia co-challenge dose before starting the experimental trial. At 13 days of age, broilers were weighed, divided into three groups (n = 15/group), and challenged with three different doses (10,000, 20,000, or 40,000) of sporulated oocysts in 1 mL volume by oral gavage. The fourth group of chicks was kept as a negative control. Five days post-challenge, body weight (BW) and body weight gain (BWG) were recorded. In the present study, challenged chickens were orally gavaged at 9:00 am with the mixed culture of EM/EA (10,000 sporulated EM containing 4% wild-type EA) at 14 days of age as this dosage reduced BWG by 35.82%. This is based on the criterion that the challenge dose must cause sub-clinical coccidiosis, consisting of a reduction between 25-35 % in BWG without the presence of clinical signs.
Animal source and experimental design
Two hundred and forty one-day-old male Cobb-Vantress broiler chickens (Fayetteville, AR, USA) were weighed and randomly allocated to one of three groups with ten replicates (n=8 chickens/replicate). Chickens were placed in battery cages, with a controlled age-appropriate environment: Group 1) Negative control (no challenge or treatment); 2) Challenge control (Eimeria challenge only); 3) Challenge + Salinomycin at 60 g/ton (BioCox 60, Huvepharma, Peachtree City, GA 30269). Chicks received ad libitum access to water and feed for 23 days. An experimental starter diet (Table 1) was formulated to approximate the nutritional requirements of broiler chickens as recommended by the National Research Council (NRC, 1994) and adjusted to breeder's recommendations (Cobb, 2015). Chickens received 23 hours of light from days 1 to 4, 20 hours of light from days 5 to 14, and 18 hours of light from days 15 to 23. Light intensity was set at 30- footcandle the first week, 1-foot candle from days eight to fourteen, and 0.5-footcandle from days 15 to 23. Temperature and light were set to mimic commercial conditions from d 1-21 in all rooms with a gradual reduction on temperature from 32 to 24°C and relative humidity at 55 ± 5%.
Performance parameters: body weight (BW), body weight gain (BWG), feed intake (FI), and feed conversion rate (FCR) were recorded at days 7, 14, 20, and 23. On day 20, half of the chickens from each replicate were weighed and euthanized while the remaining chickens were weighed and euthanized on day 23 in order to evaluate macroscopic lesions according to the scoring system of Johnson and Reid (Johnson and Reid, 1970). Oocyst per gram (OPG) was evaluated on days six, seven, and eight post-challenge, and samples were collected at 9:00 AM and 6:00 PM on each day, respectively. All animal handling procedures complied with the Institutional Animal Care and Use Committee (IACUC) at the University of Arkansas, Fayetteville. Explicitly, the IACUC approved this study under protocol #21020.
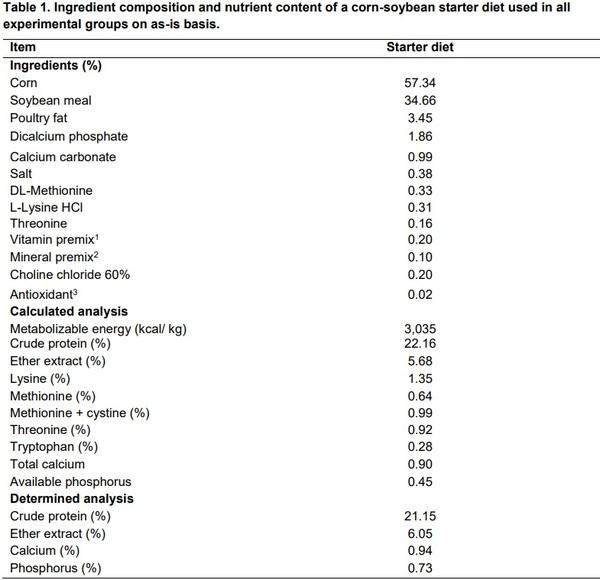
1Vitamin premix was supplied by the following per kg: vitamin A, 20,000 IU; vitamin D3, 6,000 IU; vitamin E, 75 IU; vitamin K3, 6.0 mg; thiamine, 3.0 mg; riboflavin, 8.0 mg; pantothenic acid, 18 mg; niacin, 60 mg; pyridoxine, 5 mg; folic acid, 2 mg; biotin, 0.2 mg; cyanocobalamin, 16 µg; and ascorbic acid, 200 mg (Nutra Blend LLC, Neosho, MO 64850). 2Mineral was premix supplied at the following per kg: manganese, 120 mg; zinc, 100 mg; iron, 120 mg; copper, 10 to 15 mg; iodine, 0.7 mg; selenium, 0.4 mg; and cobalt, 0.2 mg (Nutra Blend LLC, Neosho, MO 64850). 3Ethoxyquin.
Data and statistical analysis
Lesions scores, oocyst per gram, and performance data were subjected to ANOVA as a completely randomized design using the GLM procedure of SAS (SAS, 2002). For growth performance parameters (BW, BWG, FI, and FCR), each replicate cage was considered as an experimental unit. Treatment means were partitioned using Duncan's multiple range test at P<0.05 indicating statistical significance.
RESULTS AND DISCUSSION
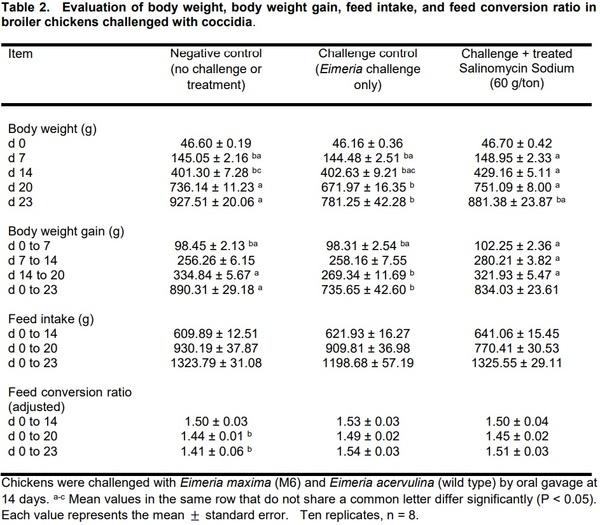
The results of the evaluation of body weight, body weight gain, feed intake, and feed conversion ratio in broiler chickens challenged with coccidia are summarized in Table 2. All three groups started with similar BW; however, at d 7, there was an increase in the BW of chickens treated with Salinomycin. By day 20 (6 days post-challenge), the negative control group (no challenged or treated) and challenge Salinomycin treated group exhibited a significant increase in BW when compared with the challenged control group (P < 0.05). Interestingly, by day 23 (9 days post-challenge), there were only significant differences in BW between the negative control group and challenge control. A similar trend was observed in BWG and FCR. No significant differences were observed in FI among the three groups (Table 2).
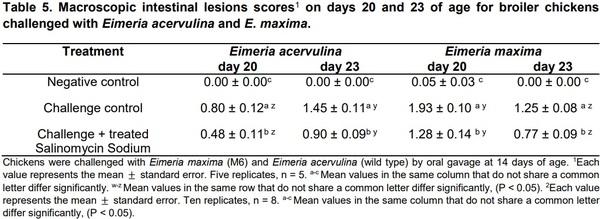
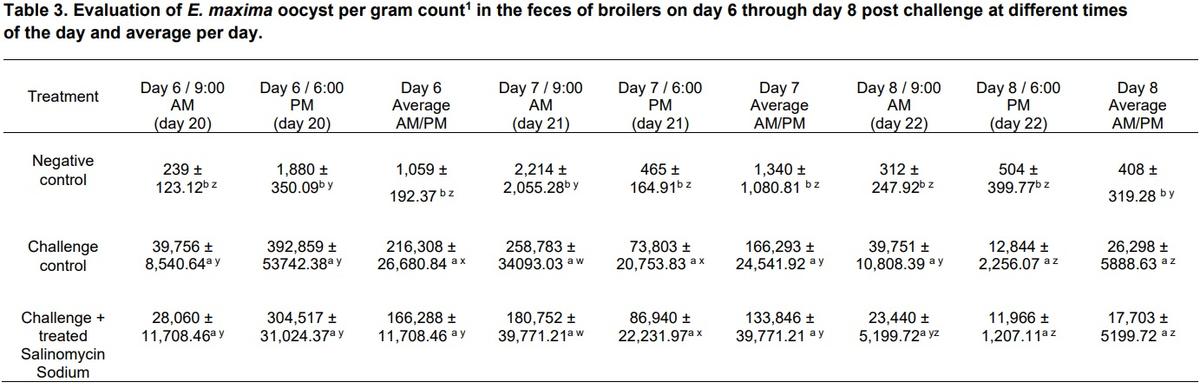
Table 3 shows the results of the evaluation of E. maxima oocyst per gram count in the feces of broilers on day 6 through day 8 post-challenge at different times of the day, the average per day. Although there was some recovery of oocysts from unchallenged untreated control chickens, there was significantly less OPG in this group compared to both challenge groups. No significant difference in OPG was observed between both challenge groups during the three days of evaluation (Table 3). In the present study, it was remarkable to find that EM oocysts were excreted in very high numbers on day 6 post-challenge in the evening for all three experimental groups. When combining and obtaining the average OPG, day 6 showed a higher number of EM oocysts, and the expected significant differences between the OPG amongst the three experimental groups were observed (Table 3). Similarly, significant differences were found in the lesion scores for EM for both evaluation days (20 and d 23) between the three experimental groups. Nevertheless, a higher number of oocysts were recovered on d 20 in both challenged groups compared to d 23 (Table 3). In the present study, negative control chickens were randomly assigned into the experimental groups that were challenged with coccidia. Perhaps, that is the reason that these chickens showed some infection, due to cross contamination of feces among the cages. Clearly, in future studies, negative control chickens must be placed in a separate room and if this is not possible, separate, and isolated cages.
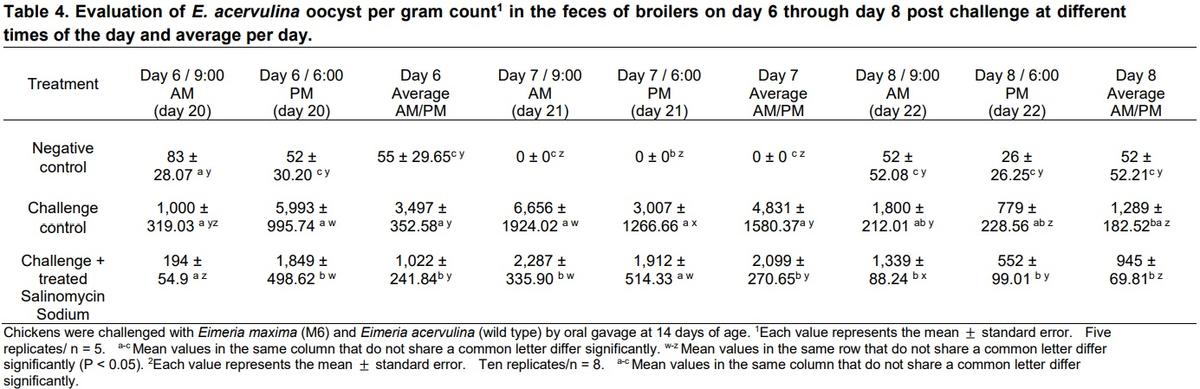
The results of the evaluation of E. acervulina oocyst per gram count in the feces of broilers on day 6 through day 8 post-challenge at different times of the day, the average per day, and lesions scores on days 20 and 23 are summarized on Table 4. A similar trend was observed in the OPG for EA, although more oocysts were present on day 23 in the challenged groups than day 20 (Table 4). Macroscopic intestinal lesions scores on days 20 and 23 of age are showed in table 5. In summary, oocyst counts were significantly different between morning and evening on d 6 post-challenge. The increased shedding of oocysts in the evening samples collections are in accordance with earlier studies of the diurnal excretion of Eimeria spp. oocysts (Hudman et al., 2000; Brown et al., 2001; Misof, 2004).
Coccidiosis remains one of the most critical diseases in poultry and results in the annual loss of millions of US dollars by the poultry industry (Williams, 2005; Chapman, 1999). One common practice in managing coccidiosis is the use of prophylactic drugs and antimicrobials that inhibit the development of sporozoites/ merozoites. However, the poultry industry is now experiencing increasing drug resistance in Eimeria strains (Abbas et al., 2012). Thus, pressure from decreasing chemical efficacy has increased demand for new treatment methods such as through plant products. As there are clear advantages to an effective controlling agent without complications with Eimeria drug resistance, there is merit in searching for effective methods with mechanisms alternative to traditional anticoccidial chemotherapeutics (Naidoo et al., 2008; Masood et al., 2013). Vaccination against coccidiosis is one alternative to chemical use. When vaccinating against coccidiosis, the natural immune system of the animal is employed to combat potential infections in the future (Shivaramaiah et al., 2014). Conventionally, live or attenuated parasites are utilized and Eimeria specific vaccines can incorporate multiple species or strains (Shivaramaiah et al., 2014). Attenuated Eimeria parasites can be selected through “precociousness,” in which “drug-sensitive, virulent strains of Eimeria spp.” are allowed to pass through a species host, reproducing, and being developed into attenuated vaccines (Peek and Landman, 2011; Shirley et al., 2007).
Previous research has described circadian variation in oocyst shedding across multiple avian host species (Hudman et al., 2000; Brown et al., 2001). Consequently, if circadian variation in oocyst shedding is not accounted for, the results of such testing are unreliable and may be misleading (Misof, 2004). A suitable method for obtaining accurate data seems to be to restrict the sampling period.
The results of this study show that the day and the time at which samples are collected can have a significant impact on data and reinforce the importance of collecting the fecal samples at the same time of day post-challenge. Oocyst counts were significantly different between morning and afternoon on day six post coccidia challenge. Coccidia load sampling should be restricted to the second half of the total daylight time. This more restrictive period should thus be considered as the preferred period for obtaining reliable information.
CONCLUSIONS
The results of this study show that the day and the time at which samples are collected can have a significant impact on data and reinforce the importance of collecting the fecal samples at the same time of day post-challenge. Coccidia load sampling should be restricted to the second half of the total daylight time. This more restrictive period should thus be considered as the preferred period for obtaining reliable information.
This article was originally published in Abanico Veterinario. January-December 2020; 10:1-11. http://dx.doi.org/10.21929/abavet2020.20.