INTRODUCTION
Hemp (Cannabis sativa L.) is an annual herbaceous plant belonging to the family Cannabinaceae1, traditionally grown for fiber and seed production. Whole hemp seed contains approximately 25% crude protein, 33-35% oil and 34% carbohydrate, in addition to a broad range of vitamins and minerals2-4. Hemp seed oil contains 75 to 80% polyunsaturated fatty acids (PUFA), including 60% linoleic acid and 17-19% α-linolenic acid (ALA)5. The nutrient composition of hemp products provides evidence that these products may serve as potentially valuable livestock feed ingredients.
In the past, the cultivation of hemp was prohibited due to the high content of Δ-9 tetrahydrocannabinol, a psychoactive substance present in the hemp plant. In the recent decades, regulatory changes undertaken by several countries across the globe allowed for the legal cultivation of industry hemp under a license that permits plants and plant parts of the genera Cannabis, the leaves and flowering heads of which do not contain more than 0.3% Δ-9 tetrahydrocannabinol (wt/wt) and includes the derivatives of such plants and plant parts. The nutritional profile, in addition to the increase in production and availability of hemp and hemp products create opportunities to use them in livestock diets6. Significant research across the globe that has gone into evaluating the safety of the ingredient showed that including hemp in animal feed is safe and offers benefits for improved animal performance and human health6,7. Initial research indicates hemp products in layers, in addition to the protein contribution, also are valuable sources of linoleic acid which is important to improve egg weight5,8,9 and linolenic acid and omega fatty acids, which have proven to have beneficial effects on human health10-12. Hemp products are also shown to be excellent sources of yolk pigmentation, lutein and fatty acid enrichment of eggs. Genetic improvements to limit Δ-9 tetrahydrocannabinol to less than 0.3% (w/w) in hemp leaves and flowering heads of the genera Cannabis, have made them safer as a feed ingredient.
The use of hemp seed cake (HSC) has not been approved in diets for any class of livestock in the USA due to a lack of adequate research in support of its safety and efficacy. The current study was designed to determine the feeding safety of HSC and its effects on systemic, tissue and organ health in commercial laying hens.
Objectives: The objectives of the study were to determine the effect of increasing levels of dietary HSC at 10, 20 and 30% on systemic, tissue and organ health, gut health and bone mineralization in commercial laying hens, as determined by:
Systemic health parameters such as blood pH, blood profile (total erythrocyte count (TEC), total leucocyte count (TLC), differential leucocyte count (DLC), packed cell volume (PCV), mean corpuscular volume (MCV), hemoglobin (Hb), mean corpuscular hemoglobin concentration (MCHC), total blood protein and serum mineral profile (Ca, P and Mg):
- Tissue and organ health parameters such as gross patho-morphology and histo-pathology of gut mucosa, spleen, duodenum, pancreas, liver and kidneys
- Gut health and environment as measured by manure quality (moisture, total nitrogen, ammonium nitrogen and mineral profile)
- Bone mineralization, as measured by fresh weight, dry weight, moisture content, ash mass and tibia bone breaking strength
MATERIALS AND METHODS
Experimental design: The study was conducted at a commercial layer farm in Lancaster County, PA. A part of the commercial layer farm was ear marked for the study and hens were organized in treatments as below. Eight hundred (800) Bovan white caged hens in lay, 30 weeks of age, were distributed in 4 treatments of 200 hens per treatment based on inclusion levels of HSC, as follows: Control diet (C0)-regular diet with no HSC, (H10)-regular diet with 10% HSC, (H20)-regular diet with 20% HSC, (H30)-regular diet with 30% HSC. Each treatment was comprised of 8 cages of 25 hens each that served as replicates. The observations per protocol were made over a period of 16 weeks following a 3 week acclimation.
Acclimation of test animals: In order to eliminate the impact of the new ingredient and its differential inclusion levels, the hens under study were subjected to a period of acclimatization for 3 weeks when the respective treatments were fed with the study diets allowing for acclimatization of feed consumption and gut environment. Observations and data from the period of acclimation were not considered for the purpose of this study.
Environment and management: All the hens under study were subjected to the following environmental and management uniformly. Special feed troughs were designed to bypass the existing auto-feeders and the hens were fed manually once a day. An iso-caloric, iso-nitrogenous diet of nutrient levels at 25lb/100 hens/day consumption as per breed standard was designed across all treatments. Continuous water, identical environment and management were offered uniformly across treatments. Hens were weighed prior to start of study by cage and composition of hens per cage was managed for uniformity of body weight across treatments. Environmental conditions were maintained at 74-76°F house temperature, 40-60% humidity, 30 Lux lighting for 15-16 h of lighting per day and air movement between 2550 and 3400 m3 h-1 per 1000 hens.
In order to establish uniformity of population across treatments, the cages were individually weighed for initial weights and, hens moved between cages so as to maintain a total body weight difference not exceeding 2.5%. These weight-adjusted cages were then randomized within the 32 cage locations with 2 cages of same treatment together. A plastic plate was installed between each cage thus preventing hens from picking feed from adjacent cage feeder.
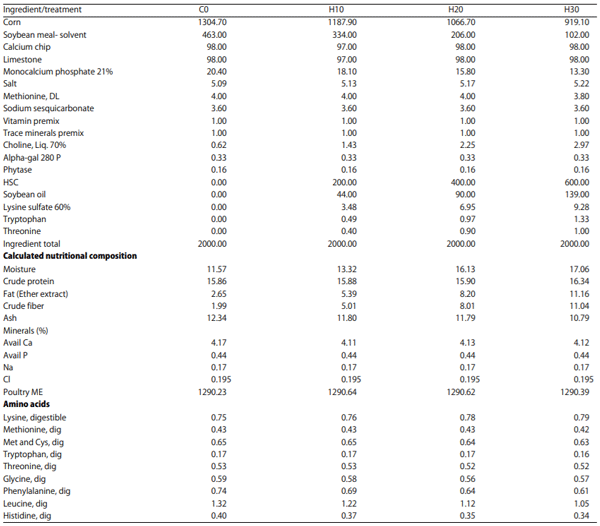
Nutritional composition of HSC and finished feed: The analysis of nutritional composition of HSC and the study diets formulated with HSC are presented in Table 1 and the formulation of the feed is presented in Table 2.
Heavy metals in HSC and study diets: The levels of heavy metals arsenic, cadmium and lead in HSC and experimental diets are reported in Table 3. The levels of heavy metals in HSC were below laboratory detectable levels. The control ration showed significantly higher levels of arsenic and cadmium over HSC diets. The lead profiles of experimental rations did not vary significantly.
Table 1: Hemp seed cake and study diet nutritional analysis (%)
Table 2: Study diets formulated by treatment (lb)
Table 3: Levels of heavy metals in HSC and study diets (mg kg-1)
Feeding program: Experimental birds were offered a uniform restricted amount of feed at 25lb/100 per day across all treatments. A pre-weighed 6.25lb of feed was provided to each cage of 25 hens every day at the same time. At this level, it was expected that the hens consumed nutrients per breed recommendation for the age and stage of production.
Preparation of composite egg sample: A specific composite sampling procedure was followed for analyzing certain parameters of egg quality, that included the following steps:
- Collect 3 eggs from each of the 8 cages of the treatment under process, a total of 24 eggs per treatment.
- Prepare 3 sets of 8 eggs each with 1 egg representing each of the cages.
- Break the 8 eggs from each set, mix and homogenize the whole egg contents for a minute with an egg homogenizer (easy mix mixer-bowl rest feature of 5 speed), pour in a sterile plastic bottle previously identified with details of treatment. This makes 1 composite sample.
- Prepare 3 such composite samples per treatment.
- Repeat the procedure for other treatments.
Study parameters, test and analytical methods: The study parameters were classified under four categories: 1. Systemic health parameters, 2. Tissue and organ health, 3. Gut health and environment and 4. Bone mineralization and were observed as follows:
Systemic health parameters: A set of systemic parameters were identified to determine the impact of feeding HSC on the general health of laying hens as any toxic, adverse or unfavorable reaction would be reflected as a change in blood profile. All blood parameters were determined on day 1, at the end of week 8 and week 16 by collecting 0.75 mL blood from the wing vein with an 18-gauge syringe needle at the rate of 1 random hen sample per cage or replicate across all treatments (n = 8 per treatment).
Blood pH: The blood pH observation was performed using a Coleman Metrion 11 pH meter equipped with standard purpose glass electrodes. Blood pH was determined on Day 1, at the end of week 8 and week 16 at the rate of 1 sample per cage across all treatments.
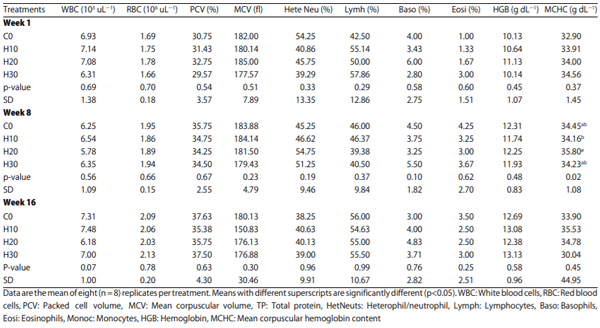
Blood profile: The complete blood count included the white blood cells (WBC), red blood cells (RBC), packed cell volume (PCV), mean corpuscular volume (MCV), total protein (TP), heterophils/neutrophils (HetNeuts), lymphocytes (Lymph), basophils (Baso), eosinophils (Eosi), monocytes (Monoc), hemoglobin (HGB), and mean corpuscular hemoglobin Concentration (MCHC) was performed.
Heparinized blood sample tubes were used to collect blood which were sent to the Avian and Exotic Animal Clinic Path Laboratory for test using the Natt and Herrick13 procedure, as follows:
Preparation of reagent:
- Dissolve 3.88 g of NaCl, 2.50 g of Na2SO4, 2.91 g of Na2HPO4-12H2O, 0.25 g of KH2PO4, 7.50 cc of Formalin (37%) and 0.10 g of Methyl Violet 2B in the order prescribed in distilled water and dilute to a total volume of 1000cc in a volumetric flask.
- Stand the solution overnight and filter through fine filter paper (Whatman No. 2). This reagent has a pH of 7.3 and ready to use.
- Count all blood cells using a Spencer "Bright-Line" Haemocytometer at a magnification of X440.
- Count TEC in all the 80 small squares.
- Count TLC in the entire central 1 mm. square (400 small squares)13.
- Calculate the percentage of each of 5 basic leucocytes (heterophils, lymphocytes, monocytes, eosinophils and basophils) as described by Çetin et al.14.
Total blood protein: The total protein was determined by using a refractometer following the method developed by Yam et al.15; briefly, 3 mL of blood were pooled from the wing vein and transferred to a heparinized coated tube from a hen per cage; the samples were centrifuged at 1358 g for 10 min at 4°C. The supernatant plasma pipetted and transferred into plastic tubes and stored at -80°C until the day of processing which occurred 3 weeks of initial plasma collection. On the day of sample processing, heparinized plasma was thawed at room temperature and diluted with 0.9% saline (NaCl) in ratio of plasma: fluid of 9:1. The refractometric assay was performed for the dilution. Plasma dilution was further aliquoted for the assay. Temperature-corrected refractometer (Reichert VET 360, Depew, NY, USA) was calibrated with de-ionized water prior to protein analysis. Protein measurements were performed in duplicate and readings were measured and recorded15.
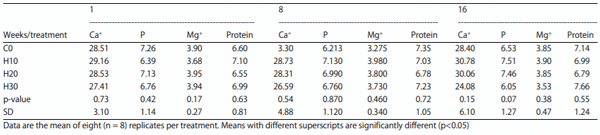
Serum mineral profile (Ca, P and Mg): Individual samples of serum from 8 hens (1 per cage = 8 per treatment), centrifuged at 15000 RPM for 10 min were sent to Veterinary Diagnostic Laboratory, University of Kentucky, Lexington KY) for calcium, phosphorus and magnesium determination using an automated clinical chemistry analyzer.
Tissue and organ health: Tissue and organ samples were taken at the end of week 16 at the rate of 1 hen per cage, or 8 hens per treatment (n = 8) and analyzed for gross patho-morphology and histo-pathology.
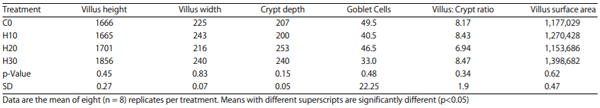
Gut mucosa: An enteric morphometric analysis was performed on a 1 cm segment from the midpoint of the duodenum. The tissues were removed and fixed in 10% buffered formalin for 72 h. The intestinal sample was then embedded in paraffin and a 2 :m section was placed on a glass slide and stained with hematoxylin and eosin for examination under a light microscope16 for villus height, villus base, villus surface area and crypt depth. Morphological parameters were measured using the Image Pro Plus v 4.5 software package. Villus height was measured from the top of the villus to the top of the lamina propria. Villus surface area was calculated using the formula
(2π) (VW/2) (VL)
Where
VW : Villus width
VL : Villus length17
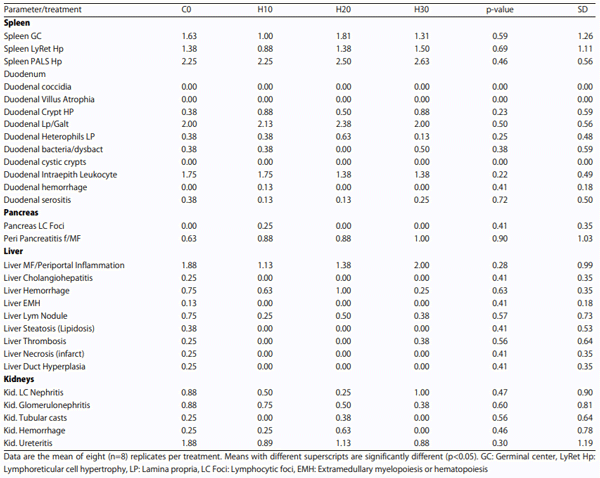
Spleen, duodenum, pancreas, liver and kidneys: Spleen, duodenum, pancreas, liver and kidney samples (n = 8) per treatment were preserved in 10% buffered formalin in plastic tubes. Organ samples were collected as 1 cm wide pieces, incised through mid-organ, placed in neutral buffered formalin for 48 h, sub-gross trimmed to 2 mm sections and routinely processed for paraffin embedded histologic sections. Five-micrometer thick paraffin sections were cut on a microtome, processed and stained with hematoxylin and eosin. Histologic examination was performed with an Olympus BX41 microscope (Olympus America, Center Valley, PA) with an attached Model MU1400 Amscope camera (Amscope, Chino, CA). The patho-morphology of the samples was performed at the Veterinary Diagnostic Pathology, LLC, Fort Valley, VA, USA for parameters as follows:
Spleen: Spleen germinal center, spleen lymphoreticular cell hypertrophy and spleen periarteriolar lymphoid sheath (T cells).
Duodenum: Duodenal coccidia, duodenal villus atrophia, duodenal crypt hypertrophy, duodenal lymphocytic infiltrates, gut associated, duodenal heterophil count, duodenal bacteriosis/dysbacteriosis, duodenal cystic crypts, duodenal inter-epithelial leucocytes, duodenal hemorrhage and duodenal serositis.
Pancreas: Pancreas lymphocytic foci and peri pancreatitis.
Liver: Liver periportal inflammation, cholangiohepatitis, liver hemorrhage, liver steatosis/lipidosis, liver lymphoid nodules, liver thrombosis, liver necrosis/infarcts, liver duct hyperplasia and liver extramedullary myelopoiesis/hematopoiesis.
Kidneys: Kidney lymphocytic foci, nephritis, glomerulonephritis, tubular casts, hemorrhage and ureteritis. The lesion panels in the report represent common lesion and tissue responses identified in chickens raised in a commercial or research environment and exposed to myriad nutritional and environmental factors. In addition, lesions that were not on these panels were noted, first as an overview examination of the study specimens and as the study progressed. These lesions are added to the panel and scored for all specimens.
Lesions (abnormal tissue, departure from normal) and tissue responses were scored semi-quantitatively to reflect a progression of standard pathology terms for the severity of the lesion: normal, minimal, mild, moderate, marked and severe. For spreadsheet recording of data, lesion scores are assigned to each term, as 0 = normal, 1 = minimal, 2 = mild, 3 = moderate, 4 = marked and 5 = severe. While specific definitions exist for every lesion, the scores generally represented the spectrum of biological response of the organ to disease, for each lesion parameter. In general, normal (0) implies the absence of a response. Trace appearance of a lesion warrants a minimal (1) score, through gradation of responses to severe (5) representing 80% or more of the biological limit for response in that organ. For this study, no actual measurements (morphometrics) were applied. Cumulative pathology was calculated by summing all lesion scores for each organ (Veterinary Diagnostic Pathology, LLC 638 South Fort Valley Road Fort Valley, Virginia 22652 USA office 540-933-6409/cell 334-750-7566).
Gut health and environment: Changes to manure parameters was considered representative of disturbances to gut environment and health, therefore, a detailed analysis of the manure was performed to determine the impact of feeding HSC treatments to laying hens. Three composite samples of fecal material per treatment were prepared by collecting samples from under each cage, thoroughly mixing the samples and dividing them into 3 composite samples per treatment. Composite samples thus prepared in sterile plastic bags were sent to Waypoint Analytical, Leola, Lancaster, PA and analyzed as follows:
Moisture: Each composite sample was dried in an oven at 95°C for 24 h or until the sample weighted constant. The moisture content was determined by the formula:
Where
WW : Wet weight
DW : Dry weight
Total nitrogen: The protein content was determined by assaying the total Nitrogen content of the excreta by using a LECO model FP 2000 N combustion analyzer (LECO Corp., St. Joseph, MI; AOAC International, 2000; method 990.03) and multiplying the result by a factor of 6.25.
Mineral profile: The mineral content was determined by using an ICP-OES (Inductively Coupled Plasma Optical Emission Spectroscopy)18.
Bone mineralization: Calcium and related mineral dynamics play a key role in laying hens and bone mineralization profile as determined by tibia bone composition and breaking strength was identified for determining the impact of feeding HSC.
Tibia composition and breaking strength (kgf g−1): The samples of tibia were collected from 1 hen from each treatment cage (8 per treatment) at the end of week 16, de-fleshed, packed in plastic bags and were overnighted to Ahpharma Research and Development Firm, Hebron, MD for determining tibia bone strength and tibia composition.
Hens were euthanized by cervical dislocation. One hen per cage was selected for the purpose and the right tibia was excised from the fresh carcass, de-fleshed without boiling. The tibiae were individually sealed in 4-oz (113.4 g) plastic bags to minimize moisture loss. The sample bags were placed into a plastic container and stored in a 4°C walk-in cooler for 1 day or a 20°C walk-in freezer for 7 days. The tibiae were dried at 105°C for 24 hours and placed in a desiccator and bone weight was recorded. Tibia breaking strength (breaking force divided by bone weight expressed as kilograms per gram) was measured using an Instron with 50 kg-load cell at 50 kg-load range with a crosshead speed of 50 mm min−1 with tibia supported on a 3.35 cm span19. Moisture free tibial ash was determined by ashing in tarred ceramic crucibles for 24 h at 615°C. The percentage of tibia ash was calculated by dividing tibia ash weights by tibia dry weight and multiplying by 100 as reported by Al-Batshan et al.20 and Park et al.21.
Statistical analysis: Systemic health parameters, tissue, organ health, gut mucosa, bone mineralization were analyzed using a completely randomized design with cage as the experimental unit with the General Linear Model Procedure (PROC GLM) of SAS (SAS Institute Inc., Cary, NC)22. The treatment mean separation were carried out with the Tukey Multiple Range test with a probability of error of 5% (p<0.05).
RESULTS
Systemic health parameters
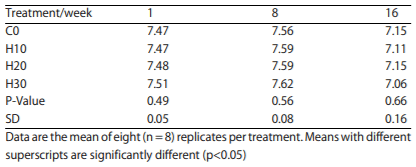
Blood pH: There was no significant difference in blood pH across all treatments, including control, during the study (Table 4).
Blood profile: The mean value of complete blood profile of hens from various treatments are presented in Table 5. Other than minor and random instances in parameters, MCHC between the 10 and 20% in week 8, no significant differences between the treatments were observed during the study.
Blood total protein and mineral profiles: The mean values of blood total protein and mineral profiles of hens fed various HSC treatments are presented in Table 6. No significant differences between the treatments were observed. Supplementation of HSC did not significantly affect the blood mineral and blood protein profiles of hens during the study.
Tissue and organ health
Gut mucosa: There was no evidence of adverse impact of feeding varying levels of HSC on gastrointestinal tissue integrity and health during the current study. Although not statistically significant, the villus height and villus surface area of H30 showed favorable trends over control and other treatments. (Table 7).
Table 4: Effect of feeding increasing levels of HSC on blood pH
Table 5: Effect of feeding increasing levels of HSC on blood profile
Table 6: Effect of feeding increasing levels of HSC on blood total protein and mineral profiles (mg dL−1)
Table 7: Effect of feeding increasing levels of HSC on gut mucosa (microns)
Spleen, duodenum, pancreas, liver, kidneys: An extensive assay of organs-spleen, duodenum, pancreas, liver and kidneys was performed in understanding of the impact of feeding HSC to laying hens at the end of study that included multiple parameters related to gross tissue morphology, histology and histopathology of spleen, duodenum, pancreas, liver and kidney. There were no significant differences among the treatments, including control (Table 8).
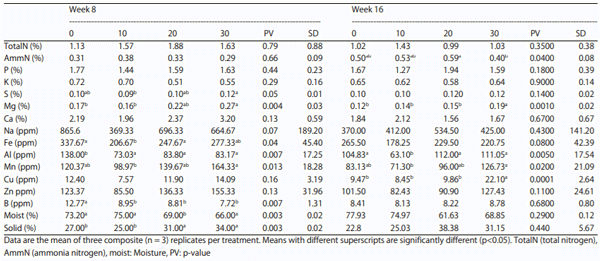
Gut health and environment: An extensive assay of manure showed a significant trend of reduction in moisture excretion over the control and a similar increasing trend in the total solids percentage with HSC feeding was noted during the first 8 weeks; however, these differences were found to be non-significant thereafter. There was no significant difference in total nitrogen excretion while ammonium nitrogen showed an inconsistent increasing trend towards end with a decline at 30% inclusion. Phosphorus, sulfur, sodium, zinc, copper and iron showed certain differences but were not found to be consistent across the treatments during the study. The levels of boron showed a reduction in excretion at week 8 in all HSC treatments compared to the control, however, was not significant across the periods of observation. Aluminum trended to reduce with HSC levels of feeding until 8 weeks and at week 16 compared to the control with 10% HSC; the same did not persist towards end of the study. A trend of increase in magnesium excretion was noticed with HSC inclusion levels, however the difference was significant with 30% HSC at both weeks 8 and 16. The H10 and H20 treatments did not show consistent differences with rest of the treatments during the study. No trends or statistical significance in excretion of calcium, phosphorous, sodium, potassium and manganese, were noted across treatments during the study (Table 9).
Table 8: Effect of feeding increasing levels of HSC on patho-morphology of spleen, duodenum pancreas, liver and kidneys
Table 9: Effect on increasing levels of HSC on manure mineral profile
Bone mineralization
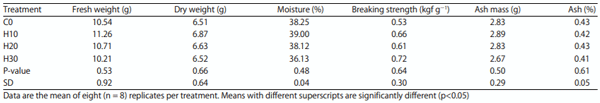
Tibial bone strength and total ash content: The nature of impact of feeding HSC on mineral metabolism in laying hens was determined by measuring the composition and breaking strength of tibial bone at the end of study. The observations revealed no impact of feeding HSC on either the composition or breaking strength of tibia. Although there were numerical differences in bone breaking strengths, no trend or statistical difference was noted. The composition parameters did not differ significantly (Table 10).
DISCUSSION
Most of the published literature on the effect of dietary HSC is in other species and with using whole hemp seed, hemp oil or other hemp products. Extremely limited published researches are available regarding the effect of feeding HSC on systemic, tissue and organ health of commercial laying hens, the authors are constrained with few supporting references to quote on the findings.
Table 10: Effect of feeding increasing levels of HSC on tibial bone parameters (kgf g−1)
Effect on systemic health: In the current study, the findings regarding the systemic health parameters strongly support the innocuous nature of the HSC as evidenced by the significant differences in blood pH, total erythrocyte count (TEC), total leucocyte count (TLC), differential leucocyte count (DLC), pack cell volume (PCV), mean corpuscular volume (MCV), hemoglobin (Hb), mean corpuscular hemoglobin concentration diet MCHC), total protein and serum calcium, serum phosphorus and magnesium levels. These findings are consistent with some previous studies conducted by Gakhar et al.6; Silversides and Lefrancois9 who studied internal physiology in laying hens.
Effect on tissue and organs: The current study unfolds the innocuous nature of HSC as evidenced by non-significant differences between control and HSC fed treatments in histo-morphological (villus height, villus width, villus depth, villus surface area, crypt depth, villus : crypt ratio and goblet cells) parameters of gut mucosa and histopathological findings of duodenum, liver, spleen, kidneys and pancreas. This finding is an addition to the current knowledge pool of safely feeding HSC to laying hens and could not be cross verified for want of related published literature.
Effect on gut health and environment: The gross impact of HSC on gut health and environment was determined by analyzing the manure for its quality during the study. The strong tendency to reducing moisture excretion and non-significant difference in total nitrogen excreted, are in favor of improving litter quality. While it could not be sufficiently backed with other findings in this study, the fact that the trend prevailed across all treatments, including control, insinuates that the HSC did not have an adverse effect on the gut physiology and environment. While the trend of excretion with the rest of minerals remained inconsistent and mostly non-significant, that of increasing trend of magnesium cannot be explained without further investigation. This finding is an addition to the current knowledge pool of safely feeding HSC to laying hens and could not be cross verified for want of related published literature.
Effect on bone mineralization: The gross effect of HSC feeding on mineral metabolism was determined by the composition and breaking strength of tibia in this study. A non-significant and inconsistent numerical differences in the fresh weight, dry weight, moisture content, ash mass, ash percentage and breaking strength of tibia was observed, it may be inferred that HSC did not interfere with bone mineralization. SkÍivan et al.23 who studied dietary hemp seed and reported that the breaking strengths of raw tibias were increased significantly (p<0.001) with all dietary concentrations of hempseed, with no difference between the experimental hempseed diet treatments. Medical research24 and experiments with rats25 showed positive effect of hempseed on bone strength of tibia. The authors opined that bone structure morphology and mesenchymal bone cell growth are possibly affected by feeding hempseed.
CONCLUSION
The current study has sufficiently evaluated and captured safety aspects of feeding HSC to commercial laying hens and concluded that dietary HSC up to 30% in layer feed did not adversely affect the systemic health. Moreover, dietary HSC up to 30% in layer feed did not have significant effect on tissues, organ health, gut health, environment and bone mineralization.
RECOMMENDATIONS
The current study explored several new areas of health, performance, toxic and residual effects of HSC for the first time and additional research may be recommended to reconfirm the findings. A further detailed study may be recommended to understand further the trend on reducing excretion moisture levels and increasing magnesium excretion while feeding HSC to commercial laying hens.
ACKNOWLEDGMENTS
The authors thankfully acknowledge Tom Beachler, Kreider Farms and Jennifer Reed-Harry, Pennsylvania Center for Poultry and Livestock for their infrastructure and financial support which made possible to successfully complete this research study.
This article was originally published in International Journal of Poultry Science, 20: 1-12. DOI: 10.3923/ijps.2021.1.12. This is an Open Access article distributed under the terms of the Creative Commons Attribution License. 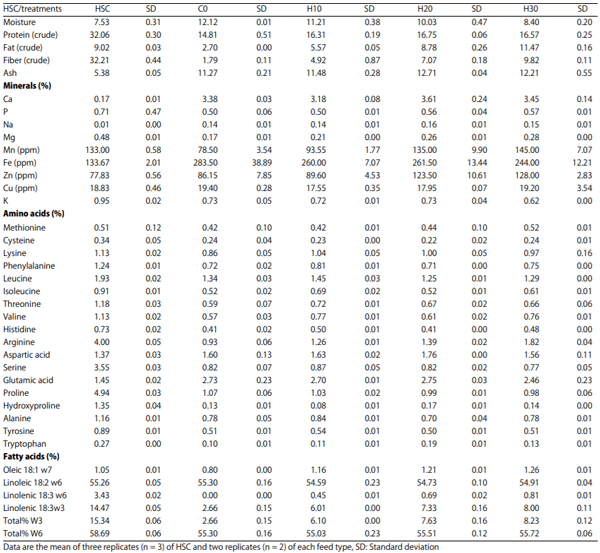

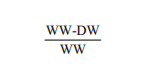










.jpg&w=3840&q=75)