Contributions of Enzyme Technology to Poultry and Swine Nutrition
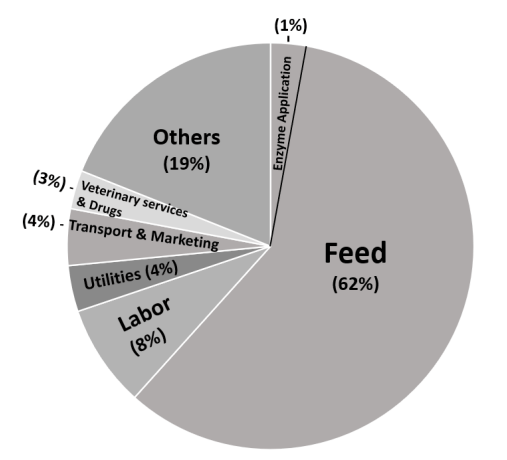
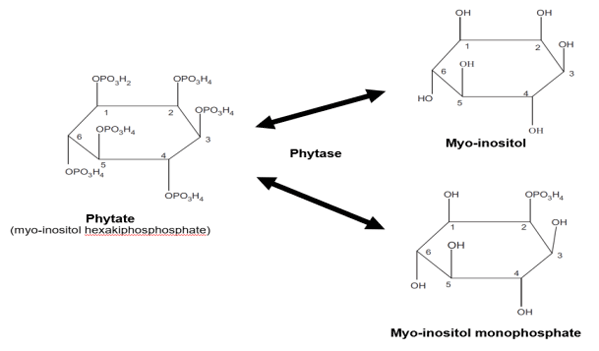
There is a widespread acceptance and encouragement of use of exogenous enzymes in poultry and swine nutrition due to their benefits. Although the benefits are unequivocal for some enzymes, for others, the benefits are inconsistent. Some of these benefits include improvements in nutrient utilization and reductions in negative effects of feedstuff-resident antinutritional factors. Feed accounts for 70% of the cost of commercial production of poultry and swine, the addition of enzymes ensures that a higher proportion of nutrients in the feed are utilized. It also encourages the use of low-cost ingredients and protects the environment by reducing the amount of nutrients present in manure. Commonly used enzymes in monogastric nutrition include phytases, carbohydrases (xylanase, β-glucanase, and amylase), and proteases. These enzymes support the intestinal hydrolysis and utilization of minerals, carbohydrates, fiber, and proteins present in feed ingredients and to varying extent have been proven to increase the production of poultry and swine. Although there are inconsistencies with some of these enzymes particularly carbohydrases and proteases, there is ongoing concerted efforts aimed at improving the efficiency of these enzymes. Genetic modifications of microbes and enzyme immobilization are some techniques being used to improve the efficacy, thermostability, specific activity, and storage stability of these enzymes. More recently, multienzyme complexes have gained increased interests in improving enzyme efficacy by capitalizing on the additive effects of the enzymes, and in reducing production cost accrued through the utilization of individual enzymes products. In conclusion, enzyme technology has contributed immensely to modern poultry and swine production and will continue to play a role in ensuring food security and sustainable agriculture.
Key words: enzymes, nutrition, poultry, swine, techniques.
Adeola, O. 2018. Phytase in starter and grower diets of White Pekin ducks. Poult. Sci. 97 592- 598.
Adeola, O., O.A. Olukosi, J.A. Jendza, R.N. Dilger, and M.R. Bedford. 2006. Response of growing pigs to Peniophora lycii- and Escherichia coli-derived phytases or varying ratios of calcium to total phosphorus. Anim. Sci. 82 637-644.
Aderibigbe, A., A.J. Cowieson, J.O. Sorbara, and O. Adeola. 2020a. Intestinal starch and energy digestibility in broiler chickens fed diets supplemented with α-amylase. Poult. Sci. 99 5907-5914.
Aderibigbe, A., A.J. Cowieson, J.O. Sorbara, and O. Adeola. 2020b. Growth phase and dietary α-amylase supplementation effects on nutrient digestibility and feedback enzyme secretion in broiler chickens. Poult. Sci. 99 6867-6876.
Aderibigbe, A., A.J. Cowieson, J.O. Sorbara, G. Pappenberger, and O. Adeola. 2020c. Growth performance and amino acid digestibility responses of broiler chickens fed diets containing purified soybean trypsin inhibitor and supplemented with a monocomponent protease. Poult. Sci. 99 5007-5017.
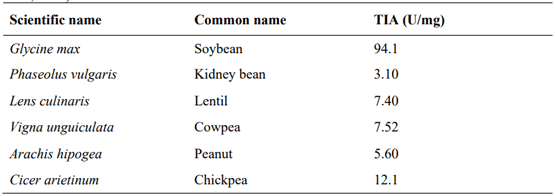
Avilés‐Gaxiola, S., C. Chuck‐Hernández, and S.O Serna Saldivar. 2018. Inactivation methods of trypsin inhibitor in legumes: a review. J. Food Sci. 83 17-29.
Babatunde, O.O., A.J. Cowieson, J.W. Wilson, and O. Adeola. 2019a. Influence of age and duration of feeding low-phosphorus diet on phytase efficacy in broiler chickens during the starter phase. Poult. Sci. 98 2588–2597.
Babatunde, O.O., A.J. Cowieson, J.W. Wilson, and O. Adeola. 2019b. The impact of age and feeding length on phytase efficacy during the starter phase of broiler chickens. Poult. Sci. 98 6742– 6750.
Babatunde, O.O., J.A. Jendza, P. Ader, P. Xue, S.A. Adedokun, and O. Adeola. 2020. Response of broiler chickens in the starter and finisher phases to three sources of microbial phytase. Poult. Sci. 99 3997–4008.
Bach Knudsen, K.E., P. Aman, and B.O. Eggum. 1987. Nutritive values of Danish-grown barley varieties. I. Carbohydrates and other major constituents. J. Cereal Sci. 6 173–186.
Barletta, A., M.R. Bedford, and G.G. Partridge. 2011. Introduction: Current market and expected developments. In Enzymes in Farm Animal Nutrition, eds. M.R. Bedford, G.G. Partridge’ Cambridge: CABI. United Kingdom. pp. 1–11.
Cherry, J.R., and A.L. Fidantsef. 2003. Directed evolution of industrial enzymes: an update. Curr. Opin. Biotechnol. 14 438-443.
Cowieson, A.J., H. Lu, K.M. Ajuwon, I. Knap, and O. Adeola. 2017. Interactive effects of dietary protein source and exogenous protease on growth performance, immune competence and jejunal health of broiler chickens. Anim. Prod. Sci. 57 252-261.
Cowieson, A. J., and F.F. Roos. 2016. Toward optimal value creation through the application of exogenous mono-component protease in the diets of non-ruminants. Anim. Feed Sci. Technol., 221 331-340.
Cowieson, A.J., J.P. Ruckebusch, I. Knap, P. Guggenbuhl, and F. Fru-Nji. 2016. Phytate-free nutrition: a new paradigm in monogastric animal production. Anim. Feed Sci. Technol., 222 180- 189.
Cowieson, A.J., M. Toghyani, S.K. Kheravii, S.B. Wu, L.F. Romero, and M. Choct. 2019. A mono-component microbial protease improves performance, net energy, and digestibility of amino acids and starch, and upregulates jejunal expression of genes responsible for peptide transport in broilers fed corn/wheat-based diets supplemented with xylanase and phytase. Poult. Sci. 98 1321-1332.
Croom, W.J., J. Brake, B.A. Coles, G.B. Havenstein, V.L. Christensen, B.W. McBride, E.D. Peebles, and I.R. Taylor. 1999. Is intestinal absorption capacity rate-limiting for performance in poultry? J. Appl. Poult. Res. 8 242– 252.
Davis, B.G. 2003. Chemical modification of biocatalysts. Curr. Opin. Biotechnol. 14 379–86.
Dhuyvetter, K.C., G.T. Tonsor, M.D. Tokach, S.S. Dritz, and J. DeRouchey. 2014. Farrowto-finish swine cost-return budget. Farm Management Guide
Dungelhoef, M., M. Rodehutscord, H. Spiekers, and E. Pfeffer. 1994. Effects of supplemental microbial phytase on availability of phosphorus contained in maize, wheat and triticale to pigs. Anim. Feed Sci. Technol. 49 1-10.
Erdaw, M.M., S. Wu, and P.A. Iji. 2017. Growth and physiological responses of broiler chickens to diets containing raw, full-fat soybean and supplemented with a high-impact microbial protease. Asian-Austr. J. Anim. Sci. 30 1303.
Evers, T., and S. Millar. 2002. Cereal grain structure and development: some implications for quality. J. Cer. Sci. 36 261-284.
Greiner, R., and U. Konietzny. 2011. Phytases: Biochemistry, enzymology, and characteristics relevant to animal feed use. In Enzymes in Farm Animal Nutrition, eds. M.R. Bedford, G.G. Partridge, Cambridge: CABI. United Kingdom. pp. 96–128.
Guisan, J., R. Fernandez-Lafuente, V. Rodriguez, A. Bastida, and G. Alvaro. 1993. Enzyme stabilization by multipoint covalent attachment to activated pre-existing supports. In: Stability and stabilization of enzymes, eds. W. van der Tweel, A. Harder, R. Buitelar, Elsevier. Amsterdam. pp. 55–62.
Guo, S., D. Liu, X. Zhao, C. Li, and Y. Guo. 2014. Xylanase supplementation of a wheat-based diet improved nutrient digestion and mRNA expression of intestinal nutrient transporters in broiler chickens infected with Clostridium perfringens. Poult. Sci. 93 94–103.
Huo, G.C., V.R. Fowler, J. Inborr, and M.R. Bedford. 1993. The use of enzymes to denature antinutritive factors in soybean. Proceedings of the Second International Workshop on Antinutritional Factors in Legume Seed, Wageningen, Netherlands. p. 60
Inborr, J., and M.R. Bedford. 1994. Stability of feed enzymes to steam pelleting during feed processing. Anim. Feed Sci. Technol. 46 179–196.
Iyer, P.V. and L. Ananthanarayan. 2008. Enzyme stability and stabilization—aqueous and nonaqueous environment. Process Biochem. 43 1019-1032.
Kalmendal, R., and R. Tauson. 2012. Effects of a xylanase and protease, individually or in combination, and an ionophore coccidiostat on performance, nutrient utilization, and intestinal morphology in broiler chickens fed a wheat-soybean meal-based diet. Poult. Sci. 91 1387–1393.
Knudsen, K. E. B., M.S. Hedemann and H.N. Lærke. 2012. The role of carbohydrates in intestinal health of pigs. Anim. Feed Sci. Technol. 173 41-53.
Le, D.M., P. Fojan, E. Azem, D. Pettersson, and N.R. Pedersen. 2013. Visualization of the anticaging effect of Ronozyme WX xylanase on wheat substrates. Cereal Chem. 90 439-444.
Lehmann, M., C. Loch, A. Middendorf, D. Studer, S.F. Lassen, L. Pasamontes, A.P. van Loon, and M. Wyss. 2002. The consensus concept for thermostability engineering of proteins: further proof of concept. Protein Eng. 15 403-411.
Leinonen, I., and A.G. Williams. 2015. Effects of dietary protease on nitrogen emissions from broiler production: a holistic comparison using Life Cycle Assessment. J. Sci. Food Agric. 95 3041-3046.
Marquardt, R.R., and M.R. Bedford. 2001. Future Horizons, In: Enzymes in Farm Animal Nutrition CABI Publishing. United Kingdom. pp. 389-398.
Menezes-Blackburn, D., and R. Greiner. 2014. Enzymes used in animal feed: leading technologies and forthcoming developments. In Functional Polymers in Food Science, eds, G. Cirillo, G. Spizzirri, F. Lemma, Wiley. Italy. pp 47−73.
Nahm, K.H. 2005. Environmental effects of chemical additives used in poultry litter and swine manure. Crit. Rev. Environ. Sci. Technol. 35 487–513.
Newkirk, R. 2010. Feed industry guide. 1st edition. Winnipeg Canada: Canadian International Grains Institute; Soybean; p. 48.
Olukosi, O.A., and O. Adeola. 2008. Whole body nutrient accretion, growth performance and total tract nutrient retention responses of broilers to supplementation of xylanase and phytase individually or in combination in wheat-soybean meal-based diets. J. Poult. Sci. 45 192–198.
Olukosi, O.A., L.A. Beeson, K. Englyst, and L.F. Romero. 2015. Effects of exogenous proteases without or with carbohydrases on nutrient digestibility and disappearance of non-starch polysaccharides in broiler chickens. Poult. Sci. 94 2662-2669.
Olukosi, O.A., A.J. Cowieson, and O. Adeola. 2007a. Age-related influence of a cocktail of xylanase, amylase, and protease or phytase individually or in combination in broilers. Poult. Sci. 86 77–86.
Olukosi, O.A., J.S. Sands and O. Adeola. 2007b. Supplementation of carbohydrases or phytase individually or in combination to diets for weanling and growing-finishing swine. J. Anim. Sci. 85 1702–1711.
Parkkonen, T., A. Tervila-Wilo, M. Hopeakoski-Nurminen, A. Morgan, K. Poutanen, and K. Autio. 1997. Changes in wheat microstructure following in vitro digestion. Acta Agric. Scand. B. Soil and Plant Sci. 47 43–47.
Petry, A.L., and J.F. Patience. 2020. Xylanase supplementation in corn-based swine diets: a review with emphasis on potential mechanisms of action. J Anim. Sci. 98 skaa318.
Rodehutscord, M. and P. Rosenfelder. 2016. Update on phytate degradation pattern in the gastrointestinal tract of pigs and broiler chickens. In Phytate destruction – consequences for precision animal nutrition eds, C.L. Walk, I. Kuhn, H.H Stein, M.T. Kidd, M. Rodehutscord, Wageningen Academic Publishers, The Netherlands. pp 15-32.
Saleh, F., A. Ohtsuka, T. Tanaka, and K. Hayashi. 2004. Carbohydrases are digested by proteases present in enzyme preparations during in vitro digestion. Jpn. Poult. Sci. 41 229–235.
Santos, T.T. C.L. Walk, P. Wilcock, G. Cordero, and J. Chewning. 2014. Performance and bone characteristics of growing pigs fed diets marginally deficient in available phosphorus and a novel microbial phytase. Can. J. Anim. Sci. 94 493–497.
Schramm, V. G., A. Massuquetto, L.S. Bassi, V.A.B. Zavelinski, J.O.B. Sorbara, A.J. Cowieson, A. P. Félix, and A. Maiorka. 2021. Exogenous α-amylase improves the digestibility of corn and corn-soybean meal diets for broilers. Poult. Sci. p101019.
Selle, P.H., and V. Ravindran. 2007. Microbial phytase in poultry nutrition. Anim. Feed Sci. Technol. 135 1–41.
Shirley, R.B., and H.M. Edwards, Jr. 2003. Graded levels of phytase past industry standards improves broiler performance. Poult. Sci. 82 671–680.
Taylor, A.E., M.R. Bedford, S.C. Pace, and H.M. Miller. 2018. The effects of phytase and xylanase supplementation on performance and egg quality in laying hens. Br. Poult. Sci. 59 554- 561
Tester, R. F., J. Karkalas, and X. Qi. 2004. Starch–Composition, fine structure, and architecture. J. Cereal Sci. 39 151–165.
Tiwari, U. P., A.K. Singh and R. Jha. 2019. Fermentation characteristics of resistant starch, arabinoxylan, and β-glucan and their effects on the gut microbial ecology of pigs: A review. Anim. Nutri. 5 217-226.
Wodzinski, R.J., and A.H.J. Ullah. 1996. Phytase. Adv. Appl. Microbiol. 42 263-303.
Wu, Y.B., V. Ravindran, D.G. Thomas, M.J. Britles, and W.H. Hendriks. 2004. Influence of phytase and xylanase, individually or in combination, on performance, apparent metabolizable energy, digestive tract measurements and gut morphology in broilers fed wheat-based diets containing adequate level of phosphorus. Br. Poult. Sci. 45 76–84.
Zhang, X., Z. Li, H. Yang, D. Liu, G. Cai, G. Li, J. Mo, D. Wang, C. Zhong, H. Wang, Y. Sun, J. Shi, E. Zheng, F. Meng, M. Zhang, X. He, R. Zhou, J. Zhang, M. Huang, R. Zhang, N. Li, M. Fan, J. Yang, and Z. Wu. 2018. Novel transgenic pigs with enhanced growth and reduced environmental impact. Elife 7:e34286.
Zuo, J., B. Ling, L. Long, T. Li, L. Lahaye, C. Yang, and D. Feng. 2015. Effect of dietary supplementation with protease on growth performance, nutrient digestibility, intestinal morphology, digestive enzymes and gene expression of weaned piglets. Anim. Nutr. 1 276-282.
Zyla, K., M. Mika, B. Stodolak, A. Wikiera, J. Koreleski, and S. Swiatkiewicz. 2004. Towards complete dephosphorylation and total conversion of phytates in poultry feed. Poult. Sci. 83 1175- 1186.



This is an excellent presentation on the use of enzymes in monogastric animal. Use of enzymes to obtain optimum performance of monogastric through maximum utilization of available nutrients in feedstuffs cannot be overemphasized. With the present situation of continuous increase in the price of feed, use of alternative feed ingredients hitherto not utilize as feedstuffs must be considered. Use of cocktail enzymes (on such alternative feedstuffs) that will enable the monogastric animal optimally utilize the available nutrients should be considered. This will reduced the cost of feed, increase return on investment for farmers and reduce environmental pollution that the alternative feedstuffs will have produced if not used for feeding animals.











