Amino Acid Nutrition and Metabolism in Chickens
Both poultry meat and eggs provide high-quality animal protein [containing sufficient amounts and proper ratios of amino acids (AAs)] for human consumption and, therefore, play an important role in the growth, development, and health of all individuals. Because there are growing concerns about the suboptimal efficiencies of poultry production and its impact on environmental sustainability, much attention has been paid to the formulation of low-protein diets and precision nutrition through the addition of low-cost crystalline AAs or alternative sources of animal-protein feedstuffs. This necessitates a better understanding of AA nutrition and metabolism in chickens. Although historic nutrition research has focused on nutritionally essential amino acids (EAAs) that are not synthesized or are inadequately synthesized in the body, increasing evidence shows that the traditionally classified nutritionally nonessential amino acids (NEAAs), such as glutamine and glutamate, have physiological and regulatory roles other than protein synthesis in chicken growth and egg production. In addition, like other avian species, chickens do not synthesize adequately glycine or proline (the most abundant AAs in the body but present in plant-source feedstuffs at low content) relative to their nutritional and physiological needs. Therefore, these two AAs must be sufficient in poultry diets. Animal proteins (including ruminant meat & bone meal and hydrolyzed feather meal) are abundant sources of both glycine and proline in chicken nutrition. Clearly, chickens (including broilers and laying hens) have dietary requirements for all proteinogenic AAs to achieve their maximum productivity and maintain optimum health particularly under adverse conditions such as heat stress and dis-ease. This is a paradigm shift in poultry nutrition from the 70-year-old “ideal protein” concept that concerned only about EAAs to the focus of functional AAs that include both EAAs and NEAAs.
Keywords Amino acids · Protein · Nutrition · Growth · Health · Egg production.
Agostinelli E (2020) Biochemical and pathophysiological properties of polyamines. Amino Acids 52:111–117 Applegate TJ, Angel R (2014) Nutrient requirements of poultry publication: history and need for an update. J Appl Poult Res 23:567–575
Asechi M, Tomonaga S, Tachibana T, Han L, Hayamizu K, Denbow DM, Furuse M (2006) Intracer-ebroventricular injection of l-serine analogs and derivatives induces sedative and hypnotic effects under an acute stressful condition in neonatal chicks. Behav Brain Res 170:71–77
Austic RE (1973) Conversion of arginine to proline in the chick. J Nutr 103:999–1007
Awad EA, Zulkifli I, Farjam AS, Chwen LT (2014) Amino acids fortification of low-protein diet for broilers under tropical climate. 2. Nonessential amino acids and increasing essential amino acids. Ital J Anim Sci 13:3297
Badawi MS, Ali AH, El-Razik WMA, Soliman MH (2019) Influence of low crude protein diets on broiler chickens performance. Adv Anim Vet Sci 7:26–33
Bailey CA (2020) Precision poultry nutrition and feed formulation. In: Bazer FW, Lamb GC, Wu G (eds) Animal agriculture: challenges, innovations, and sustainability. Elsevier, New York, pp 367–378
Baker DH (1997) Ideal amino acid profiles for swine and poultry and their applications in feed formulation. BioKyowa Tech Rev 9:1–24
Baker DH (2009) Advances in protein-amino acid nutri-tion of poultry. Amino Acids 37:29–41
Baker DH, Han Y (1994) Ideal amino acid profile for chicks during the first three weeks posthatching. Poult Sci 73:1441–1447
Baker DH, Sugahara M, Scott HM (1968) The glycine-serine interrelationship in chick nutrition. Poult Sci 47:1376–1377
Belloir P, Méda B, Lambert W, Corrent E (2017) Reduc-ing the CP content in broiler feeds: impact on animal performance, meat quality and nitrogen utilization. Animal 11:1881–1889
Calderon VM, Jensen LS (1990) The requirement for sulfur amino acid by laying hens as influenced by the protein concentration. Poult Sci 69:934–944
Chrystal PV, Moss AF, Khoddami A, Naranjo VD, Selle PH, Liu SY (2020) Effects of reduced crude protein levels, dietary electrolyte balance, and energy density on the performance of broiler chickens offered maize-based diets with evaluations of starch, protein, and amino acid metabolism. Poult Sci 99:1421–1431
Coon C, Balling R (1984) Asparagine and glutamine metabolism in chicks. Poult Sci 63:717–729
Corzo A, Fritts CA, Kidd MT, Kerr BJ (2005) Response of broiler chicks to essential and non-essential amino acid supplementation of low crude protein diets. Anim Feed Sci Technol 118:319–327
Curthoys NP, Watford M (1995) Regulation of glutamin-ase activity and glutamine metabolism. Annu Rev Nutr 15:133–159
Dean WF, Scott HM (1965) The development of an amino acid reference diet for the early growth of chicks. Poult Sci 44:803–808
Dessimoni GVI, Dalólio FS, Moreira J, Teixeira LV, Bertechini AGV (2019) Protease supplementation under amino acid reduction in diets formulated with different nutritional requirements for broilers. Braz J Poult Sci 21:1–8
Dong X, Yang C, Tang S, Jiang Q, Zou X (2010) Effect and mechanism of glutamine on productive perfor-mance and egg quality of laying hens. Asian Australas J Anim Sci 23:1049–1056
Emadi M, Jahanshiri F, Kaveh K, Hair-Bejo K, Ideris A, Alimon AR (2011) Nutrition and immunity: the effects of the combination of arginine and tryptophan on growth performance, serum parameters and immune response in broiler chickens challenged with infectious bursal disease vaccine. Avian Pathol 40:63–72
Erwan E, Tomonaga S, Yoshida J, Nagasawa M, Ogino Y, Denbow DM, Furuse M (2012) Central administration of L- and D-aspartate attenuates stress behaviors by social isolation and CRF in neonatal chicks. Amino Acids 43:1969–1976
Erwan E, Chowdhury VS, Nagasawa M, Goda R, Otsuka T, Yasuo S, Furuse M (2014) Central injection of L- and D-aspartate attenuates isolation induced stress behavior in chicks possibly through different mechanisms. Eur J Pharmacol 736:138–142
Fang YZ, Yang S, Wu G (2002) Free radicals, antioxidants, and nutrition. Nutrition 18:872–879
Fisher H, Scott HM (1954) The essential amino acid requirements of chicks as related to their proportional occurrence in the fat-free carcass. Arch Biochem Biophys 51:517–519
Flynn NE, Wu G (1996) An important role for endogenous synthesis of arginine in maintaining arginine homeo-stasis in neonatal pigs. Am J Physiol 271:R1149– R1155
Fouad AM, Ei-Senousey HK, Yang XJ, Yao JH (2013) Dietary L-arginine supplementation reduces abdomi-nal fat content by modulating lipid metabolism in broiler chickens. Animal 7:1239–1245
Fu WJ, Haynes TE, Kohli R, Hu J, Shi W, Spencer TE, Carroll RJ, Meininger CJ, Wu G (2005) Dietary L-arginine supplementation reduces fat mass in Zucker diabetic fatty rats. J Nutr 135:714–721
Furukawa K, He WL, Leyva-Jimenez H, Bailey CA, Bazer FW, Toyomizu M, Wu G (2018) Developmental changes in the activities of enzymes for polyamine synthesis in chickens. Poult Sci 97(E-Suppl 1):3–4
Furukawa K, Kikusato M, Wu G, Toyomizu M (2020) Suppressive effects of glutamate and glutamine co-supplementation on muscle proteolysis in heat-stressed broiler chickens. Poultry Science Association Annual Meeting
Gholipour V, Chamani M, Shahryar HA, Sadeghi A, Afshar MA (2017) Effects of dietary L-glutamine sup-plement on performance, egg quality, fertility and some blood biochemical parameters in Guinea fowls (Numida meleagris). Kafkas Univ Vet Fak Derg 23:903–910
Gilbert ER, Li H, Emmerson DA, Webb KE Jr, Wong EA (2010) Dietary protein composition influences abun-dance of peptide and amino acid transporter messenger ribonucleic acid in the small intestine of 2 lines of broiler chicks. Poult Sci 89:1663–1676
Glista WA, Mitchell HH, Scott HM (1951) The amino acid requirements of the chick. Poult Sci 30:915
Graber G, Baker DH (1973) The essential nature of gly-cine and proline for growing chickens. Poult Sci 52:892–896
Hamasu K, Haraguchi T, Kabuki Y, Adachi N, Tomonaga S, Sato H, Denbow DM, Furuse M (2009) L-Proline is a sedative regulator of acute stress in the brain of neonatal chicks. Amino Acids 37:377–382
Hamasu K, Shigemi K, Tsuneyoshi Y, Yamane H, Sato H, Denbow DM, Furuse M (2010) Intracerebroventricular injection of l-proline and d-proline induces sedative and hypnotic effects by different mechanisms under an acute stressful condition in chicks. Amino Acids 38:57–64
Haraguchi T, Tomonaga S, Kurauchi I, Hamasu K, Sato H, Denbow DM, Furuse M (2007) Intracerebroventricular injection of l-proline modifies food intake in neonatal chicks. J Anim Vet Adv 6:1255–1257
He WL, Wu G (2020) Metabolism of amino acids in the brain and their roles in regulating food intake. Adv Exp Med Biol 1265:167–185
He WL, Furukawa K, Leyva-Jimenez H, Bailey CA, Wu G (2018) Oxidation of energy substrates by enterocytes of 0- to 42-day-old chickens. Poult Sci 97(E-Suppl 1):3
Herring CM, Bazer FW, Wu G (2020) Amino acid nutri-tion for optimum growth, development, reproduction, and health of zoo animals. Adv Exp Med Biol 1285:233-253
Hou YQ, Wu G (2017) Nutritionally nonessential amino acids: a misnomer in nutritional sciences. Adv Nutr 8:137–139
Hou YQ, Wu G (2018) L-Glutamate nutrition and metab-olism in swine. Amino Acids 50:1497–1510
Hou YQ, Wu ZL, Dai ZL, Wang GH, Wu G (2017) Protein hydrolysates in animal nutrition: industrial pro-duction, bioactive peptides, and functional signifi-cance. J Anim Sci Biotechnol 8:24
Hou YQ, He WL, Hu SD, Wu G (2019) Composition of polyamines and amino acids in plant-source foods for human consumption. Amino Acids 51:1153–1165
Hu H, Bai X, Shah AA, Wen AY, Hua JL, Che CY, He SJ, Jiang JP, Cai ZH, Dai SF (2016a) Dietary supplemen-tation with glutamine and γ-aminobutyric acid improves growth performance and serum parameters in 22- to 35-day-old broilers exposed to hot environ-ment. J Anim Physiol Anim Nutr 100:361–370
Hu H, Bai X, Wen A, Shah AA, Dai S, Ren Q, Wang S, He S, Wang L (2016b) Assessment of interactions between glutamine and glucose on meat quality, AMPK, and glutamine concentrations in pectoralis major meat of broilers under acute heat stress. J Appl Poult Res 25:370–378
Huston RL, Scott HM (1968) Effect of varying the com-position of crystalline amino acid mixture on weight gain and pattern of free amino acids in chick tissue. Fed Proc 27:1204–1209
Izumi T, Kawamura K, Ueda H, Bungo T (2004) Central administration of leucine, but not isoleucine and valine, stimulates feeding behavior in neonatal chicks. Neurosci Lett 354:166–168
Jobgen WJ, Meininger CJ, Jobgen SC, Li P, Lee MJ, Smith SB, Spencer TE, Fried SK, Wu G (2009) Dietary L-arginine supplementation reduces white-fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats. J Nutr 139:230–237
Johnson CA, Duong T, Latham RE, Shirley RB, Lee JT (2020) Effects of amino acid and energy density on growth performance and processing yield of mixed-sex Cobb 700 MV broiler chickens. J Appl Poult Res 29:269–283
Kawakami S-I, Bungo T, Ohgushi A, Ando R, Shimojo M, Masuda Y, Denbow DM, Furuse M (2000) Brain-derived mast cells could mediate histamine-induced inhibition of food intake in neonatal chicks. Brain Res 857:313–316
Klain G, Johnson BC (1962) Metabolism of labeled aminoethanol, glycine, and arginine in the chick. J Biol Chem 237:123–126
Klain GJ, Scott HM, Johnson BC (1960) The amino acid requirements of the growing chick fed a crystalline amino acid diet. Poult Sci 39:39–44
Kluess HA, Stafford J, Evanson KW, Stone AJ, Worley J, Wideman RF (2012) Intrapulmonary arteries respond to serotonin and adenosine triphosphate in broiler chickens susceptible to idiopathic pulmonary arterial hypertension. Poult Sci 91:1432–1440
Lemme A (2009) Amino acid recommendations for laying hens. Lohmann Inf 44:21–32
Leveille GA, Fisher H (1959) Amino acid requirements for maintenance in the adult rooster. II The requirements for glutamic acid, histidine, lysine and arginine. J Nutr 69:289–294
Li P, Wu G (2018) Roles of dietary glycine, proline and hydroxyproline in collagen synthesis and animal growth. Amino Acids 50:29–38
Li P, Wu G (2020) Composition of amino acids and related nitrogenous nutrients in feedstuffs for animal diets. Amino Acids 52:523–542
Li P, Kim SW, Nakagawa K, Zhou HJ, Wu G (2010) Dietary supplementation with L-glutamine and AminoGut™ enhances protein synthesis in skeletal muscle of growing broiler chickens. FASEB J 24:740.21
Li XL, Rezaei R, Li P, Wu G (2011) Composition of amino acids in feed ingredients for animal diets. Amino Acids 40:1159–1168
Li XL, Zheng SX, Wu G (2020a) Nutrition and metabo-lism of glutamate and glutamine in fish. Amino Acids 52:671–691
Li WW, Yang JC, LYU QF, Wu GF, Lin SM, Yang QG, Hu JM (2020b) Taurine prevents cardiomyocyte apo-ptosis by inhibiting the calpain-1/cytochrome c path-way during RVH in broilers. Amino Acids 52:453–463 Liu SY, Selle PH, Raubenheimer D, Cadogan DJ, Simpson SJ, Cowieson AJ (2016) An assessment of the influence of macronutrients on growth performance and nutrient utilisation in broiler chickens by
nutritional geometry. Br J Nutr 116:2129–2138 Maruyama K, Sunde ML, Harper AE (1976) Is L-glutamic
acid nutritionally a dispensable amino acid for the young chick? Poult Sci 55:45–60
Matthews JC (2000) Amino acid and peptide transport system. In: Farm animal metabolism and nutrition. JPF D’Mello CAPI Publishing, Wallingford, pp 3–23
McNeill SH, Belk KE, Campbell WW, Gifford CL (2017) Coming to terms: meat’s role in a healthful diet. Anim Front 7:34–42
Mund MD, Riaz M, Mirza MA, Rahman Z, Mahmood T, Ahmad F, Ammar A (2020) Effect of dietary trypto-phan supplementation on growth performance, immune response and antioxidant status of broiler chickens from 7 to 21 days. Vet Med Sci 6:48–53
Nakashima K, Yakabe Y, Ishida A, Yamazaki M, Abe H (2007) Suppression of myofibrillar proteolysis in chick skeletal muscles by a-ketoisocaproate. Amino Acids 33:499–503
Nakashima K, Yakabe Y, Ishida A, Katsumata M (2008) Effects of orally administered glycine on myofibrillar proteolysis and expression of proteolytic-related genes of skeletal muscle in chicks. Amino Acids 35:451–456
NRC (National Research Council) (1994) Nutrient requirements of poultry, 9th edn. National Academy Press, Washington, DC
Osmanyan AK, Ghazi Harsini S, Mahdavi R, Fisinin VI, Arkhipova AL, Glazko TT, Kovalchuk SN, Kosovsky GY (2018) Intestinal amino acid and peptide transporters in broiler are modulated by dietary amino acids and protein. Amino Acids 50:353–357
Oso AO, Williams GA, Oluwatosin OO, Bamgbose AM, Adebayoc AO, Olowofeso O, Pirgozliev V, Adegbenjo AA, Oshoe SO, Alabi JO, Li F, Liu H, Yao K, Xin W (2017) Effect of dietary supplementation with arginine on haematological indices, serum chemistry, carcass yield, gut microflora, and lymphoid organs of growing turkeys. Livest Sci 198:58–64
Ospina-Rojas IC, Murakami AE, Oliveira CAL, Guerra AFQG (2013) Supplemental glycine and threonine effects on performance, intestinal mucosa develop-ment, and nutrient utilization of growing broiler chickens. Poult Sci 92:2724–2731
Pirsaraei ZA, Rahimi A, Deldar H, Sayyadi AJ, Ebrahimi M, Shahneh AZ, Shivazad M, Tebianian M (2017) Effect of feeding arginine on the growth perfor-mance, carcass traits, relative expression of lipogenic genes, and blood parameters of Arian broilers. Braz J Poult Sci 20:363–370
Porteous JW (1980) Glutamate, glutamine, aspartate, asparagine, glucose and ketone-body metabolism in chick intestinal brush-border cells. Biochem J 188:619–632
Price WA Jr, Taylor MW, Russell WC (1953) The reten-tion of essential amino acids by the growing chick. J Nutr 51:413–422
Reeds PJ, Burrin DG, Stoll B, Jahoor F (2000) Intestinal glutamate metabolism. J Nutr 130:978S–982S
Refaie AM, Abdallah AG, Khosht AR, Magied HAA, Habib HH, Waly AH, Shaban SAM (2017) Response of broiler chicks to low-protein-L-valine supplemented diets formulated based on digestible amino acids. J Anim Poult Prod 8:13–19
Réhault-Godbert S, Guyot N, Nys Y (2019) The Golden egg: nutritional value, bioactivities, and emerging benefits for human health. Nutrients 11:684
Robel E, Menge H (1973) Performance of chicks fed an amino acid profile diet based on carcass composition. Poult Sci 52:1219–1221
Sasse CE, Baker DH (1973) Modification of the Illinois reference standard amino acid mixture. Poult Sci 52:1970–1972
Sestili P, Martinelli C, Colombo E, Barbieri E, Potenza L, Sartini S, Fimognari C (2011) Creatine as an antioxi-dant. Amino Acids 40:1385–1396
Smith DD, Campbell JW (1983) Subcellular location of chicken brain glutamine synthetase and comparison with chicken liver mitochondrial glutamine synthetase. J Biol Chem 258:12265–12268
Tan BE, Yin YL, Liu ZQ, Li XG, Xu HJ, Kong XF, Huang RL, Tang WJ, Shinzato I, Smith SB, Wu G (2009) Dietary L-arginine supplementation increases muscle gain and reduces body fat mass in growing-finishing pigs. Amino Acids 37:169–175
Tan JZ, Guo YM, Applegate TJ, Du EC, Zhao X (2014) Dietary L-arginine modulates immunosuppression in broilers inoculated with an intermediate strain of infectious bursa disease virus. J Sci Food Agric 95:126–135
Tinker DA, Brosnan JT, Herzberg GR (1986) Interorgan metabolism of amino acids, glucose, lactate, glycerol and uric acid in the domestic fowl (Gallus domesticus). Biochem J 240:829–836
Tomonaga S, Tachibana T, Takagi T, Saito E-S, Zhang R, Denbow DM, Furuse M (2004) Effect of central administration of carnosine and its constituents on behaviors in chicks. Brain Res Bull 63:75–82
Tomonaga S, Kaji Y, Tachibana T, Denbow DM, Furuse M (2005) Oral administration of b-alanine modifies carnosine concentrations in the muscles and brains of chickens. Anim Sci J 76:249–254
Tran PV, Chowdhury VS, Do PH, Bahry MA, Yang H, Furuse M (2016) L-Ornithine is a potential acute sati-ety signal in the brain of neonatal chicks. Physiol Behav 155:141–148
Tran PV, Chowdhury VS, Furuse M (2019) Central regu-lation of feeding behavior through neuropeptides and amino acids in neonatal chicks. Amino Acids 51:1129–1152
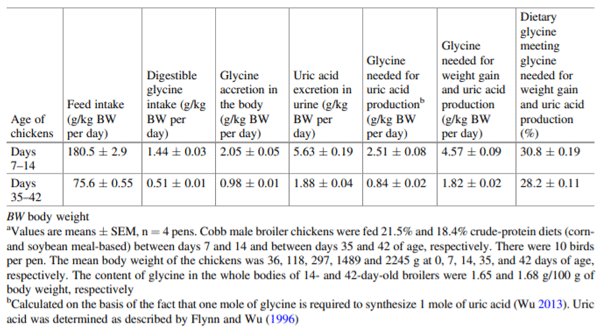
Wang WW, Wu ZL, Dai ZL, Yang Y, Wang JJ, Wu G (2013) Glycine metabolism in animals and humans: implications for nutrition and health. Amino Acids 45:463–477
Wang WW, Dai ZL, Wu ZL, Lin G, Jia SC, Hu SD, Dahanayaka S, Wu G (2014) Glycine is a nutritionally essential amino acid for maximal growth of milk-fed young pigs. Amino Acids 46:2037–2045
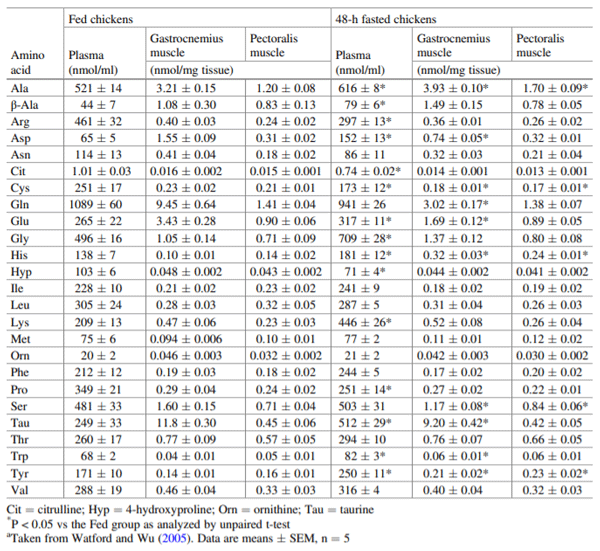
Watford M, Wu G (2005) Glutamine metabolism in urico-telic species: variation in skeletal muscle glutamine synthetase, glutaminase, glutamine levels and rates of protein synthesis. Comp Biochem Physiol B Biochem Mol Biol 140:607–614
Watford M, Hod Y, Chiao YB, Utter MF, Hanson RW (1981) The unique role of the kidney in gluconeogene-sis in the chicken. The significance of a cytosolic form of phosphoenolpyruvate carboxykinase. J Biol Chem 256:10023–10027
Wen C, Wu P, Chen YP, Wang T, Zhou YM (2014) Methionine improves the performance and breast mus-cle growth of broilers with lower hatching weight by altering the expression of genes associated with the insulin-like growth factor-I signaling pathway. Br J Nutr 111:201–206
Winiarska-Mieczan A, Kwiecień M, Kwiatkowska K, Baranowska-Wójcik E, Szwajgier D, Zaricka E (2020) Fatty acid profile, antioxidative status and die-tary value of the breast muscle of broiler chickens receiving glycine-Zn chelates. Anim Prod Sci 60:1095–1102
Wu G (2009) Amino acids: metabolism, functions, and nutrition. Amino Acids 37:1–17
Wu G (2010) Functional amino acids in growth, reproduc-tion and health. Adv Nutr 1:31–37
Wu G (2013) Amino acids: biochemistry and nutrition. CRC Press, Boca Raton
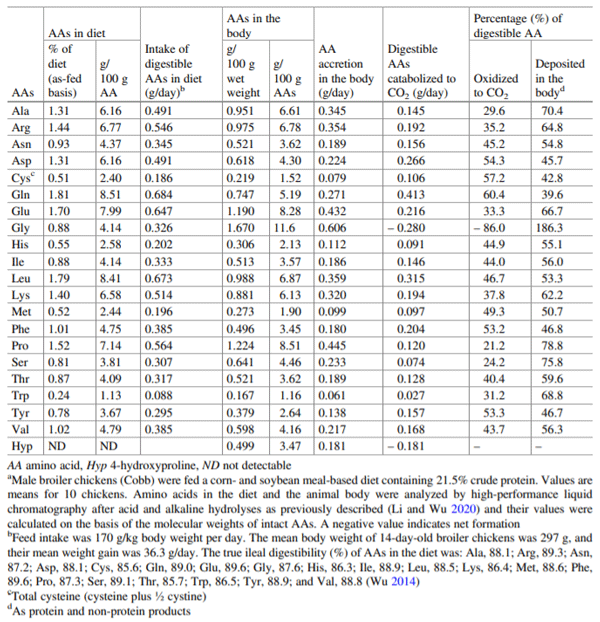
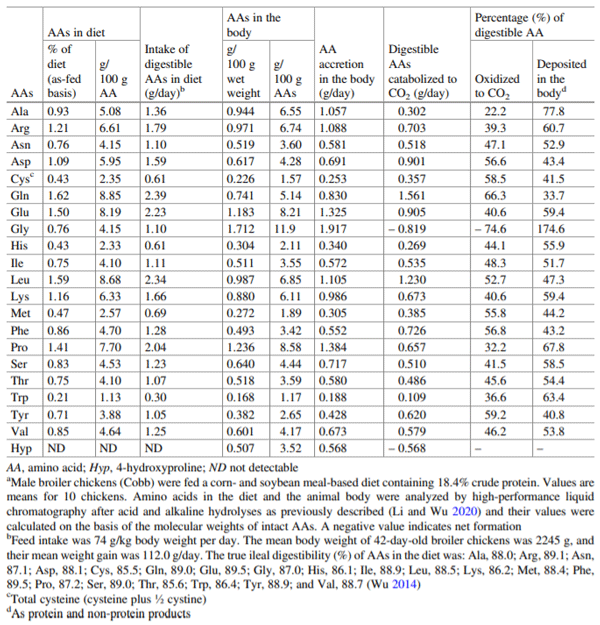
Wu G (2014) Dietary requirements of synthesizable amino acids by animals: a paradigm shift in protein nutrition. J Anim Sci Biotechnol 5:34
Wu G (2018a) Principles of animal nutrition. CRC Press, Boca Raton
Wu G (2018b) Functional amino acids in poultry nutrition: recent developments and practical applications. Annual Meeting of Poultry Science Association, San Antonio. July 23–26, 2018
Wu G (2020a) Management of metabolic disorders (including metabolic diseases) in ruminant and nonru-minant animals. In: Bazer FW, Lamb GC, Wu G (eds) Animal agriculture: challenges, innovations, and sustainability. Elsevier, New York, pp 471–492
Wu G (2020b) Important roles of dietary taurine, creatine, carnosine, anserine and hydroxyproline in human nutrition and health. Amino Acids 52:329–360
Wu G, Thompson JR (1987) Ketone bodies inhibit leucine degradation in chick skeletal muscle. Int J Biochem 19:937–943
Wu G, Thompson JR (1990) The effect of glutamine on protein turnover in chick skeletal muscle in vitro. Biochem J 265:593–598
Wu G, Thompson JR, Sedgwick G, Drury M (1989) For-mation of alanine and glutamine in chick (Gallus domesticus) skeletal muscle. Comp Biochem Physiol 93B:609–613
Wu G, Thompson JR, Baracos VE (1991) Glutamine metabolism in skeletal muscle from the broiler chick (Gallus domesticus) and the laboratory rat (Rattus norvegicus). Biochem J 274:769–774
Wu G, Borbolla AG, Knabe DA (1994) The uptake of glutamine and release of arginine, citrulline and proline by the small intestine of developing pigs. J Nutr 124:2437–2444
Wu G, Flynn NE, Yan W, Barstow DG (1995) Glutamine metabolism in chick enterocytes: absence of pyrroline-5-carboxylate synthase and citrulline synthesis. Biochem J 306:717–721
Wu G, Chung-Bok M, Vincent N, Kowalski TJ, Choi YH, Watford M (1998) Distribution of phosphate-activated glutaminase isozymes in the chicken: absence from liver but presence of high activity in pectoralis muscle. Comp Biochem Physiol B 120:285–290
Wu G, Bazer FW, Burghardt RC, Johnson GA, Kim SW, Knabe DA, Li P, Li XL, McKnight JR, Satterfield MC, Spencer TE (2011a) Proline and hydroxyproline metabolism: implications for animal and human nutri-tion. Amino Acids 40:1053–1063
Wu G, Bazer FW, Johnson GA, Knabe DA, Burghardt RC, Spencer TE, Li XL, Wang JJ (2011b) Important roles for L-glutamine in swine nutrition and production. J Anim Sci 89:2017–2030
Wu ZL, Hou YQ, Dai ZL, Hu CA, Wu G (2019) Metabo-lism, nutrition and redox signaling of hydroxyproline. Antioxid Redox Signal 30:674–682
Wu G, Bazer FW, Lamb GC (2020) Significance, challenges and strategies of animal production. In: Bazer FW, Lamb GC, Wu G (eds) Animal agriculture: challenges, innovations, and sustainability. Elsevier, New York, pp 1–20
Xiao M, Mi YL, Liu LJ, Lv CF, Zeng WD, Zhang CQ, Li J (2018) Taurine regulates mucosal barrier function to alleviate lipopolysaccharide-induced duodenal inflam-mation in chicken. Amino Acids 50:1637–1646
Yamane H, Tsuneyoshi Y, Denbow DM, Furuse M (2009a) N-methyl-D-aspartate and a-amino-3-hydroxy-5-methyl-4isoxazolepropionate receptors involved in the induction of sedative effects under an acute stress in neonatal chicks. Amino Acids 37:733–739
Yamane H, Asechi M, Tsuneyoshi Y, Kurauchi I, Denbow DM, Furuse M (2009b) Intracerebroventricular injec-tion of L-aspartic acid and L-asparagine induces seda-tive effects under an acute stressful condition in neonatal chicks. Anim Sci J 80:286–290
Yasugi S, Mizuno T (1981) Developmental changes in acid proteases of the avian proventriculus. J Exp Zool A 216:331–335
Yazdanabadi FI, Moghaddama GH, Nematollahib A, Daghighkiaa H, Sarirc H (2020) Effect of arginine sup-plementation on growth performance, lipid profile, and inflammatory responses of broiler chicks challenged with coccidiosis. Prev Vet Med 180:10503
Yoshida J, Tomonaga S, Ogino Y, Nagasawa M, Kurata K, Furuse M (2012) Intracerebroventricular injection of kynurenic acid attenuates corticotrophin-releasing hormone-augmented stress responses in neo-natal chicks. Neuroscience 220:142–148
Zarghi H, Golian A, Nikbakhtzade M (2020) Effect of dietary digestible lysine level on growth performance, blood metabolites and meat quality of broilers 23–38 days of age. J Anim Physiol Anim Nutr 104:156–165 Zhai W, Peebles ED, Wang X, Gerard PD, Olanrewaju HA, Mercier Y (2016) Effects of dietary lysine and methionine supplementation on Ross 708 male broilers from 21 to 42 d of age (III): serum metabolites, hormones, and their relationship with growth performance. J Appl Poult Res 25:223–231
Zhang S, Saremi B, Gilbert ER, Wong EA (2017a) Physi-ological and biochemical aspects of methionine isomers and a methionine analogue in broilers. Poult Sci 96:425–439
Zhang Y, Zhao L, Zhou Y, Diao C, Han L, Yinjie N, Liu S, Chen H (2017b) Glutamine ameliorates mucosal dam-age caused by immune responses to duck plague virus. Dose-Response. https://doi.org/10.1177/15593258177 08674














