Antimicrobial Resistance - the Link between Animals and Man
Published: November 2, 2017
By: David G.S. Burch.
/ Octagon Services Ltd, The Round House, The Friary, Old Windsor, Berkshire, SL42NR, United Kingdom.
Introduction
Antimicrobial resistance and its potential transmission from animals to man has become a major issue, both politically and scientifically and is leading to greater controls, both in Europe and North America in particular, on how we use antibiotics in agriculture and veterinary medicine. There is deep and sincere concern expressed by the medical profession about the worsening antimicrobial resistance situation in man and the potential that agricultural/ veterinary use of antimicrobials may be adding to their problem to a large extent? Hence there is a call for “One Health” approach between human and animal use of antibiotics to try to combat the problem. However, much of the proposed legislation and controls on veterinary medicine is not based on factual assessments but assumptions, and the contribution that agricultural use is making on human antimicrobial resistance problems has not been quantified. This paper attempts to quantify the significance of the use of antimicrobials in pigs and poultry on human antimicrobial resistance in the EU which will enable a model to be developed to determine it on a national basis.
Overview of resistance development and spread
The use of antibiotics, especially when given by mouth, either in feed or in drinking water or tablets etc may have a direct effect on the bacteria in the gut i.e. kills them off if they are susceptible. They may be good bacteria (lactobacilli) or the bad pathogenic bacteria that one is trying to treat like Escherichia coli or Brachyspira hyodysenteriae. This exposure may select for organisms that are either inherently resistant, so they don´t die, then they multiply because of reduced competition or selects for resistant bacteria that have already acquired resistance. Bacteria can acquire resistance either by mutations of their DNA (remember they are often growing and multiplying at a very fast rate) and if this mutation is on chromosomes this leads to clonal spread as the bacteria multiply e.g. Campylobacter spp and fluoroquinolone resistance. Sometimes they acquire resistance via plasmids, which are passed from one cell to another by conjugation (almost sexually) and some bacteria like E. coli can spread plasmids very readily and these carry potentially resistant genes so are spread horizontally to possibly susceptible bacteria or potentially other bacterial species. Less common routes of transmission are by transformation, they pick up DNA left by other bacteria or by transduction where bacteriophages (viruses) inject the DNA. After treatment, the gut flora stabilises over time and often returns to what it was before. Some bacteria that have acquired resistant genes or plasmids do not always survive or compete very well (reduced fitness) and naturally die off. Hopefully, after exposure to the infection and treatment the animal has developed immunity or is resistant to further infections.
Generally, in pig medicine a lot of oral antimicrobials via feed or drinking water are used; hence, many of the bacteria found in the gut carry a higher level of resistance. Resistance is often higher in young or weaned pigs where they have been commonly treated but by the time they go for slaughter (5-6 months of age) the resistance is usually less. This is important to reduce the potential indirect transmission of resistance via meat contamination. In broiler production, more water medication is used and birds are slaughtered at 5-6 weeks of age, hardly giving time for the gut flora to become reestablished. Clonal fluoroquinolone resistant C. jejuni are also commonly present.
Oral antibiotics are commonly used to treat respiratory diseases such as Mycoplasma hyopneumoniae (enzootic pneumonia), Actinobacillus pleuropneumoniae, or systemic diseases like Streptococcus suis (strep meningitis) of Haemophilus parasuis poultry we have M. gallisepticum, M. synoviae and Avibacterium paragallinarum but the most common bacterial systemic invader is E. coli. Generally, resistance is lower in these respiratory infections but the gut flora is exposed at the same time, hence tetracycline resistance is very high in E. coli (around 80%) in pigs but relatively low in A. pleuropneumoniae (24%).
However, the main public concern about resistance in pigs and poultry, is the potential transference of resistance, whether via zoonotic bacteria, which can colonise and cause disease in man such as Salmonella or Campylobacter spp and make them more difficult to treat. Commensal bacteria such as E. coli and Enterococcus spp may or may not colonise the human gut but may transmit resistance via plasmids and genes to the human gut flora. Usually, they do not cause disease directly in man.
Staphylococcus aureus has become a recent concern, as it can colonise a pig´s nose and may spread to man. In many countries in the EU, N. America and Asia many pig farms carry the methicillin resistant form (MRSA) in particular CC398 and CC9 in China. The bacterium has there quite happily and may not cause disease, however, it can colonise a human nose also but usually for a short time. It can cause disease in man but hospitals that screen patients are very worried about it coming into the wards and being spread to other patients or contaminating wounds of the carriers post surgery.
Direct spread
The direct spread of MRSA from pigs to man has been a major EU issue. Ninety percent of MRSA CC398 human carriers and infected patients in Denmark (1) were associated with pig farming, either workers/farmers or veterinarians and their families. In Germany, colonisation was reported at 83% in pig farmers and 4.3% of their families (2). Additionally, 36% of pig veterinarians and 14% of slaughterhouse workers (3) had nasal colonisation. The spread beyond to the general population was very limited. The methicillin-susceptible form (MSSA) has been around for a long time in humans but somehow got into pigs and it is postulated that the widespread use of 3rd generation cephalosporins in the 2000´s probably selected for MRSA in piggeries. Methicillin or related compounds are not used in pig medicine but once it has the mecA gene it is resistant to all beta-lactam (penicillin-based) antibiotics. The pig associated MRSA is usually tetracycline resistant and has been found to have a chromium/zinc resistance cra gene associated with the mecA gene. Both tetracyclines and zinc oxide are widely used in many pig-producing countries but these are not primary selectors of methicillin resistance but may be co-selectors, if they have the resistance genes as well as they may kill off susceptible bacteria, enhancing the survival of the resistant bacteria.
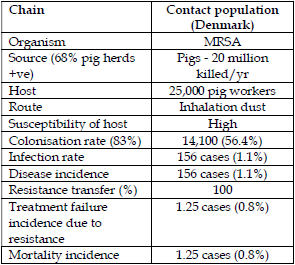
In Denmark, there is great concern about the spread of MRSA from pigs to man. The Danes (4) identified that in 2013, 68% of finisher herds were MRSA positive and colonisation with MRSA CC398 in man was increasing rapidly to 643 cases (30.7%) of overall MRSA colonized patients and infection and clinical disease associated with CC398 was 156 cases (16.8%) and of these, bacteraemias were 1.8% and actual deaths were lower at approximately 0.8%. All mortalities had a number of serious underlying diseases. The potential direct spread of the pig MRSA to stockmen can be very high and therefore methicillin resistance spread to stockmen is also high. By comparison, the spread amongst the general Danish population is incredibly low and potential infection rate is 0.0028% in comparison with 1.1% in stockmen.
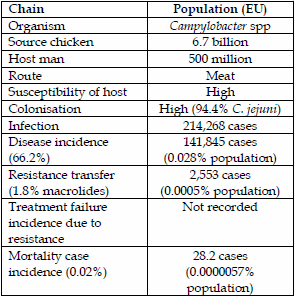
| Table 1. Epidemiology of direct contact infection MRSA Denmark. | Table 2. Campylobacter spp transmission by chickens to humans and macrolide resistance. | |
 |  |
Similarly, Streptococcus suis caused high infection rates with 21% of stockmen being seropositive in the UK (5) but were all penicillin sensitive. Four percent of E. coli were shown to have similar resistance profiles between farmers and their pigs in the Netherlands (6).
Indirect spread
Campylobacter spp are currently the most frequently transmitted enteric infections transmitted from animals to man, mainly by contaminated food and the environment (7). Campylobacter jejuni infections are the most common in man accounting for approximately 94.4% and C. coli for approximately 5.6% (8) in a Dutch case control study. Chickens have a similar proportion of Campylobacter species to humans and cattle are predominantly C. jejuni too. Pigs however, carry predominantly C. coli. Carcass contamination of pork with Campylobacter spp is also very low at 0.6% but chicken carcasses are high at 31% (9). The Dutch (8) looked at a combined case control and genetic source attribution analysis for both C. jejuni and C. coli in the Netherlands, using multilocus sequence typing (MLST). Overall, they attributed cases, 66.2% to chicken, cattle 20.7%, environment 10.1%, sheep 2.5%, and pigs only 0.3%.
The resistance of C. jejuni from chicken meat to the fluoroquinolone, ciprofloxacin, in the EU is relatively high at 59.5% but the resistance to macrolides (erythromycin) is comparatively low at 1.8% (10). In contrast, the resistance of porcine C. coli from pork is lower (32%) but erythromycin higher (23.9%).
The attribution of macrolide resistance transmission from chickens to man was 0.0005% or 0.5 people/100,000 population. The attribution of disease transmission from pigs is 0.3% or 643 cases and macrolide resistance transfer from pigs is 23.9% or 154 cases. On an EU population basis this represents 0.00003% of cases or 0.03 people/100,000 population, which is also very low. This is in accord with the European Medicines Agency´s categorisation of macrolides (11) as a lower risk family of antimicrobials in their Category 1, in contrast to the World Health Organisation´s (12) assessment of being a Highly Critically Important Antibiotic (HCIA).
Fluoroquinolone resistance (59.5%) transmission by campylobacter from chickens is much higher and involves 84,398 cases or 0.0169% on a population basis or 16.9 people/100,000 population. However, this is mainly a chromosomal/clonal resistance so is unlikely to be transmitted to other bacteria. In pigs with the fluoroquinolone resistance at 32%, this would be in 206 campylobacter cases and only 0.00004% of the EU population or 0.04 people/100,000 population.
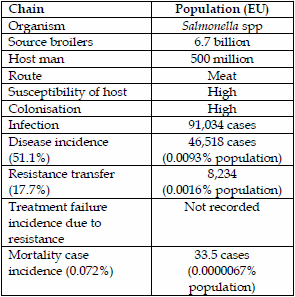
Salmonella spp. cases in man have been steadily falling in the EU since 2004 when it was 195,947 cases (13) until 2012 when it was 91,034 cases (7), a 54% fall, following the introduction of vaccine and hygiene measures in poultry flocks. The main effect has been a major reduction of the incidence of S. enterica Enteritidis, which contaminated meat and eggs but S. Typhimurium cases, the main pig isolate, have stayed much the same.
The main human salmonella serovars were 41.3% S. Enteritidis and 29.3% S. Typhimurium and monophasics (7). Pigs are commonly associated with S. Typhimurium but phage typing of GB isolates tells a different story that possibly only one third (9.8%) are pig associated (14) and therefore 51.1% are still chicken associated (both S. Enteritidis and S. Typhimurium). Pig isolates of S. Typhimurium showed a 7.5% resistance to fluoroquinolones and chicken isolates were 17.7%. With regard to 3rd generation cephalosporins pig isolates were 2.3% and chicken isolates were 4.0%, respectively.
The disease attribution from chickens to man of fluoroquinolone resistance is 8,234 cases or 0.0016% of the EU population or 1.6 people /100,000 population. Regarding pigs to man transmission of fluoroquinolone resistance it is estimated at 687 cases or 0.00014% on a population basis or 0.14people/100,000 population, which is really quite low.
Cefotaxime or 3rd generation cephalosporin resistance transmission primarily due to ESBLs from pig salmonella to man are summarized below.
| Table 3. Salmonella spp transmission of fluoroquinolone resistance from chickens to man. | Table 4. Salmonella spp transmission of cephalosporin resistance (ESBLs) from pigs to man. | |
 | 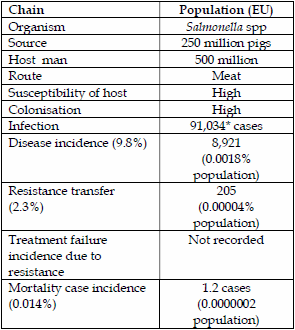 |
The estimated transmission rate of cefotaxime (3G) or ESBL resistance via Salmonella spp from pigs to man is 0.00004% or 0.04 people/100,000 population. For chickens the transmission rate is higher at 0.00037% or 0.37 people/100,000 population. On this basis ESBL resistance transmission can be considered very small even for chickens.
This has been supported by recent work (15, 16, 17 & 18) in the EU looking genetically at ESBL resistance genes found in urinary tract infections and blood-borne infections in man and comparing them from ESBLs found in animals and food.
| Table 5. Combined results ESBL resistance gene attribution from animals and food and those in clinical infections in man. | Table 6. Comparison of risk assessments for transfer of infectious agents and antibiotic resistance directly from pigs to farmers or indirectly from meat to man. | |
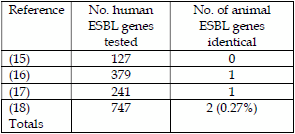 |  |
These results demonstrated that 2/747 (0.27%) ESBL resistant genes were identical to genes found in animals and food and that 745/747 (99.73%) were attributable to human use of 3rd and 4th generation cephalosporins in man. Surprisingly, the Danes concluded in their Report that “consumption of meat may currently be considered an insignificant source for the humans infections”. Using the Swedish data (16) the attribution rate of ESBLs from animals to human infections was 1/379 (0.26%), which represents on a transmission rate basis that the number of clinical cases potentially caused by ESBL containing E. coli from food/farm animals = 21.2/8,161 cases or an infection rate of 0.00022%/year on a 9.5 million Swedish population basis. This represents 0.22 people/100,000 population out of 85 people/100,000 population which would normally get infected, i.e. an extremely low infection rate.
Risk assessment summary
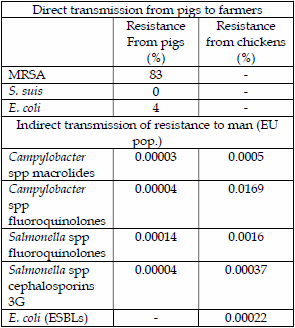
A comparison of risk assessments for transfer of antibiotic resistance from pigs and chickens to man are summarised below.
The main contrast is between direct contact with animals causing high percentages of infection and potential resistance transfer and the indirect route via meat causing fractional percentages of resistance transfer. Chicken meat seems to be a higher risk than pork both due to carcass contamination, especially by campylobacter and also by higher fluoroquinolone resistance. Somewhat surprisingly, both macrolide and cephalosporin resistance transfer from both chicken meat and pork are comparatively low.
Conclusions
It can be seen from these calculations that direct transmission of infections and potentially antimicrobial resistance is very common between farmers and their animals. In contrast, the indirect route via food actually transmits very low levels of resistance. Care must be taken that the reported EU figures of cases are only those that have been submitted and that potentially these underestimate the number of cases of diarrhoea that are not reported and treated at home, but it does represent the severer cases that have visited the doctor and are more likely to have been treated. A factor of 10 might be used to compensate for unreported cases, but even so, this is an extremely low rate.
Poultry meat appears to be a higher risk of causing disease than pig meat, possibly due to greater carcass contamination and also the use of fluoroquinolones in the drinking water, usually to treat systemic E. coli infections is likely to select for resistant clones of C. jejuni and Salmonella spp.
Living and working with animals is likely to present a higher risk of obtaining antimicrobial resistance. The overuse of antibiotics can also increase the incidence of antibiotic resistance and therefore the use of these vital medicines must be responsibly and not just to compensate for poor husbandry. It is felt that the attribution of antimicrobial resistance in man caused by the indirect contact with meat products that are poorly cooked is often over emphasized in human health and the major contributor to human resistance issues are the direct use of antibiotics in man, where in the US it is calculated that 16.3% of the population were treated with antibiotics each year (19).
Acknowledgements
Sincere thanks to Prof. Dr. Padet Tummaruk of Chulalongkorn University for inviting me to speak. Financial support for my visit and presentation was kindly provided by Elanco Animal Health.
Presented at the Chulalongkorn University Veterinary Conference 2016. This article was originally published in Thai Journal of Veterinary Medicine Supplement, 2016, 46: 57-62.
References
1. DANMAP 2010. 2011. Report-Antibiotics Denmark.
2. Cuny C et al., 2009. PLOS One, August: pp e6800.
3. Blaha T et al., 2009. Proc. 14th ISAH Congress Vechta, Germany, vol 2, pp.645- 648.
4. DVFA. 2014. Report-MRSA Dec. 2014.
5. Barlow AM et al., 2003. Pig J., 51: 164-176.
6. Nijsten R et al., 1996. J Antimicrobial Chemotherapy, 37: 1131-1140.
7. EFSA/ECDC. 2014a. EFSA J., 12(2): 3547.
8. Mughini Gras L et al., 2012. Plos One, 7(8): e42599.
9. EFSA/ECDC. 2011. EFSA J., 9(3): 2090.
10. EFSA/ECDC. 2014b. EFSA J., 12(2): 3590.
11. EMA. 2014. EMA/381884/2014.
12. WHO. 2011. Report-Critical Antibiotics.
13. EFSA/ECDC. 2010. EFSA J., 1496.
14. AHVLA. 2014. Report-Salmonella.
15. Wu GH et al., 2013. PLOS One, 8(9): e75392.
16. SVARM 2014. 2015. Report-Antibiotics Sweden.
17. DANMAP 2014. 2015. Report-Antibiotics Denmark.
18. Burch DGS, 2015. Vet Rec., 177: 549-550.
19. Burch, DGS. 2013. Pig J., 68: 27-39.
Related topics:
Authors:
Octagon Service
Recommend
Comment
Share

Would you like to discuss another topic? Create a new post to engage with experts in the community.






.jpg&w=3840&q=75)