Lessons Learned on Virus Transmission: A Retrospective View of the Canada PEDV Outbreak
Published: July 8, 2020
By: Dr. Louis E. Russell
A newly published open access review article [1] discusses the epidemiological conclusions made about how porcine epidemic diarrhea virus (PEDV) may have been introduced to the first confirmed PEDV case farm in Ontario, Canada on January 22, 2014. The authors noted that an epidemiological investigation [2] had concluded that “The weight of evidence gathered during an outbreak of porcine epidemic diarrhea (PED) in Canada in January 2014 supports an association with feed containing spray dried porcine plasma contaminated with the virus” and discussed that in retrospect, much has been learned about critical factors related to the spread of PEDV and that the conclusions of previous investigations could be different if these critical factors had been considered.
Some of the factors discussed are summarized as follows:
The index farm case may not represent the first introduction of PEDV into Canada
- On January 21, 2014, a pork packer in Quebec reported a positive PEDV genome environmental sample and confirmed that the trucks delivering hogs to that plant did not come from the US.
- On January 22, 2014, the index case was confirmed on a pig farm in Ontario.
- On January 24, 2014 there were 10 environmental samples collected at an Ontario assembly yard that were confirmed positive for PEDV genome.
If the index farm case did not bring PEDV to Ontario how could PEDV have been introduced?
Contaminated animal transport truck movement: The Canadian epidemiology did not include an assessment of animal transport movements at the time of their investigations. Today it is known that trucks returning from packing plants represent a high risk of being contaminated with PEDV genome, so current truck wash protocols use extensive multiple steps to decontaminate trucks to prevent the spread of PEDV. However, at the time of the initial PEDV case in Ontario, trucks were routinely transporting Canadian pigs to PEDV contaminated US packing plants and returning directly to Canada under regulations that only required the trucks to be cleaned of visible manure using a shovel and broom before returning to Canada. This is a more logical explanation for the wide-spread environmental PEDV contamination found at the Ontario assembly yard and at the Quebec packing plant.
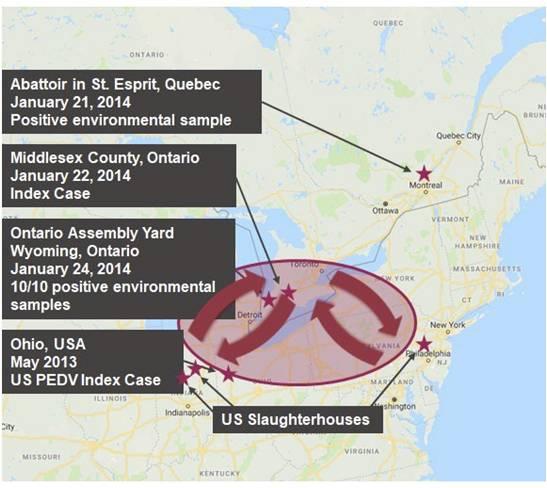
Contaminated feed mill trucks and non-animal feed ingredients: The Canadian epidemiology did not include an assessment of the feed mill biosecurity protocols at the time of their investigation. Since 2014, research has shown that feed mills can be contaminated with PEDV from trucks delivering feed ingredients or feed mill trucks delivering feed to swine farms and returning to the feed mill. It is possible that feed mill biosecurity protocols in place at the time of the Canadian PEDV outbreak were not as rigorous as those recommended today and post-processing PEDV contamination of the feed or feed delivery trucks could have contributed to the PEDV outbreak. Also, since 2014, research has shown that the PEDV can survive for longer periods of time on conventional soybean meal than other feed ingredients, including animal origin feed ingredients. During 2014, Canada imported over a million-metric ton of soybean meal from the United States. Contaminated non-animal feed ingredients infecting market hogs could be an alternative explanation of a potential source of the PEDV-positive environmental samples at Quebec abattoir and the Ontario assembly yard.
How could the plasma sample collected by the CFIA become contaminated with infective virus?
The FDA pig bioassays [3] did not detect infective PEDV in the manufacturer retained sample from the lot of spray dried porcine plasma (SDPP) investigated by CFIA. However, the sample of SDPP that CFIA collected at the feed mill supplying nursery feed to the index case farm was tested in a pig bioassay [4] and was contaminated with infective PEDV. Around the same time of the index case, all bags of the remaining SDPP inventory at the feed manufacturer were sampled multiple times, initially by the feed mill QA personnel and then by OMAFRA officials before the CFIA collected the SDPP sample used in their bioassay. If feed biosecurity and sampling protocols were not as rigorous as those in place today, environmental contamination from the feed manufacturing facility, equipment, or multiple sampling of the same bag(s) creates the potential for prior contamination of the SDPP sample CFIA collected and used in their bioassay.
Since the time of the index case other research has demonstrated that PEDV inoculated on SDPP does not survive over 1–3 weeks, depending upon storage temperatures. The SDPP investigated by CFIA was produced over 10 weeks before the index case and over 13 weeks before CFIA collected the sample used in their bioassay [5]. These data support the hypothesis that infectious PEDV -contamination of the SDPP sample collected by CFIA occurred after the product left control of the SDPP manufacturer, either during transport or during storage and use at the feed mill.
FDA did not detect infective PEDV in the retained samples or a breach of Good Manufacturing Practices of the SDPP product investigated in the Canada PEDV outbreak.
Epidemiologists investigating the Canadian PEDV outbreak suggested that a breach in good manufacturing practices (GMPs) could result in PEDV contamination of the SDPP being investigated. The FDA reviewed manufacturing records of the lot of SDPP investigated by CFIA and did not identify a breach of GMPs or preventive controls [3].
The WHO guidelines for assuring the viral safety of human blood products recommend that the production process include processing steps able to inactivate 4 logs of non-enveloped and enveloped viruses. The SDPP manufacturing process follows WHO recommendations and has been shown to inactivate > 4 logs of both envelope and non-enveloped viruses and selected pathogenic bacteria. In addition, published trials report that pigs fed PCR-positive SDPP at high levels in the feed and for extended periods of time did not become infected. This confirms that while SDPP may contain PCR positive virus genome, PCR-positive test results do not imply infectivity. This information confirms that the manufacturing process is robust and that SDPP is a safe feed ingredient.
In the months prior to the Canadian PEDV outbreak, the plasma manufacturer regularly exported SDPP PCR positive for PEDV to Brazil and to Western Canada from the same production facility produced under the same GMPs as the lot of SDPP investigated by CFIA. The amount of SDPP exported during this period was enough to feed 2.5–3.5 million pigs in Brazil and 3.5–4.0 million pigs in Western Canada. Neither region experienced a PEDV outbreak during that time period.
Summary
The Canadian epidemiological investigation about the index farm PEDV case in Canada was extensive but did not include several critical factors that may have changed the conclusions.
- It is likely that PEDV was present in Eastern Canada prior to the confirmed index farm case.
- It is likely that minimal cleaning of trucks returning from PEDV-contaminated US slaughter plants resulted in the introduction of PEDV into Eastern Canada and led to contamination of the Ontario assembly yard and the Quebec packing plant.
- It is likely that animal truck movement between the contaminated Ontario assembly yard, other common sites, and the initial PEDV infected farms contributed to the spread of PEDV.
- There is no support for the suggestion that a breach in GMPs was responsible for the infective PEDV that CFIA reported on the SDPP sample they collected.
- Multiple sampling of all bags of the SDPP at the feed mill could have contaminated the sample of SDPP tested by the CFIA with infectious PEDV.
- The manufacturing process for SDPP includes validated inactivation steps using GMPs and follows WHO safety guidelines for human plasma transfusion.
- OIE recognizes that SDPP is a safe feed ingredient when GMPs are followed.
References
- Russell, L.E, J. Polo and D. Meeker. 2020. The Canadian 2014 porcine epidemic diarrhea virus outbreak: Important risk factors that were not considered in the epidemiological investigation could change the conclusions. Transbound Emerg Dis. 2020;00:1–12; https://doi.org/10.1111/tbed.13496
- Aubry, P, J.L. Thompson, T. Pasma, M.C. Furness and J. Tataryn. 2016. Weight of the evidence linking feed to an outbreak of porcine epidemic diarrhea in Canadian swine herds. J. Sw. Health and Prod. 25(2)69-72.
- NASDBPP. 2014a. Controlled experiments provide conclusive evidence spray-dried porcine plasma is a safe ingredient and is not a source of infective PED virus. www.aasv.org/pedv/research/NASDBPP research.pdf.
- Pasick, J., Berhane, Y., Ojkic, D., Maxie, G., Embuty-Hyatt, C., Swekla, K., Alexandersen, S. (2014). Investigation into the role of potentially contaminated feed as a source of the first-detected outbreaks of porcine epidemic diarrhea in Canada. Transboundary and Emerging Diseases, 61, 397–410. https://doi.org/10.1111/tbed.12269.
- NASDBPP. 2014b. Studies point to plasma as a safe feed ingredient. Feedstuffs. 2014:86(28):10-11.16.
Related topics:
Authors:
APC, Inc.
Influencers who recommended :
Joe CrenshawRecommend
Comment
Share

Would you like to discuss another topic? Create a new post to engage with experts in the community.






