Fusarium toxins
Fumonisins - Mycotoxins of increasing importance!
Published: August 12, 2008
By: DI Karin Griessler, Technical Manager - BIOMIN GmbH (Austria)

Fumonisins are a group of recently discovered mycotoxins which belong to the family of Fusarium toxins. The contamination of feedstuffs with mycotoxins poses a serious threat to the health and productivity of animals and cause great economic losses. In the USA, the annual losses caused by mycotoxins in grain production are estimated at 900 million dollars (CAST 2003). Dependent on type of animal, sex, age as well as the nutritional and health condition of the animal, fumonisins cause different clinical symptoms. Additionally, the occurrence of several mycotoxins in feed is very likely and can amplify the toxic effects of the individual toxin (synergistic effect).
Fumonisins are mainly produced by Fusarium verticillioides (syn. moniliforme) as well as by Fusarium proliferatum (see Figure 1) and they occur predominately in maize and maize-based feeds (Ross et al. 1992). In 1988 they were first identified and isolated and so far there are 28 fumonisin analogues known (Gelderblom et al. 1988; Rheeder et al. 2002). Fumonisins are divided into four groups: Serial A, B, C and G. With regard to their toxicity the B-type fumonisins represent the most important ones (Marasas 1996). In naturally contaminated food and feed fumonisin B1 represents about 70% - 80% of the total fumonisin content (Krska et al. 2007).
Fumonisins are very polar and water soluble compounds. Unlike other mycotoxins they have a long chain structure. Chemically they are polyhydroxyl alkylamines esterified with two carbon acids, i.e. tricarballylic acid (TCA). The four common members of the type B fumonisins differ by presence and position of the free hydroxyl groups respectively (ApSimon 2001). The one-sided or bilateral elimination of TCA results in partial hydrolyzed fumonisin or hydrolyzed fumonisin (HFB1).
Fungal colonization and growth and/or mycotoxin production are influenced by a variety of factors. Optimum conditions for fumonisin production are temperatures between 10°C and 30°C with a water activity (amount of free available water) of 0.93 aw (Marin et al. 1999).
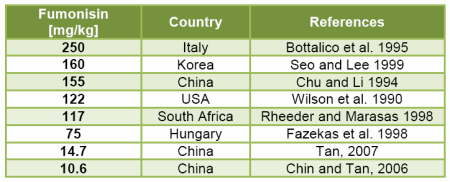
A recently published survey about the occurrence of mycotoxins in Asia initiated by Biomin GmbH together with Romer® laboratories in Singapore reported that 58% out of 678 feed raw material samples were contaminated with fumonisins. The highest level of fumonisins detected was 14.7 mg/kg in a corn sample from China (Tan, 2007). In Europe, the maximum level of fumonisin was 3.1 mg/kg in a sample of soybean meal from Southern Europe (Binder et al. 2007). Table 1 gives examples on high concentrations of fumonisins in maize.

Figure 1: Fusarium proliferatum contaminated maize (Source: Chamber of Agriculture, Styria, Austria)
Table 1: Examples on high concentrations of fumonisins in maize samples
Toxicity
Fumonisin toxicity is based on the structural similarity to the sphingoid bases, sphingosine and sphinganine (see Figure 2). They are inhibitors of sphinganine (sphingosine) Nacyltransferase (ceramide synthase), a key enzyme in the lipid metabolism, resulting in a disruption of this pathway. This enzyme catalyzes the acylation of sphinganine in the biosynthesis of sphingolipids and also the deacylation of dietary sphingosine and the sphingosine that is released by the degradation of complex sphingolipids (ceramid, sphingomyelin and glycosphingolipide) (Wang et al. 1991). Sphingolipids are basically important for the membrane and lipoprotein structure and also for cell regulations and communications (second messenger for growth factors) (Berg et al. 2003).
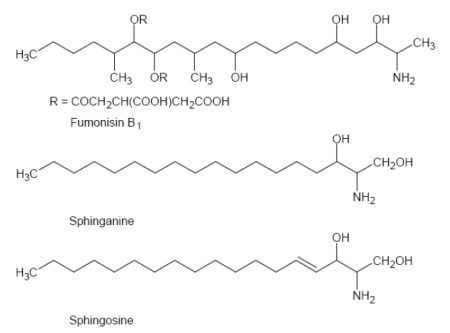
Figure 2: Structures of sphinganine, sphingosine and fumonisin B1.
As a consequence of this disruption many bioactive intermediates are elevated, others reduced. The main points are:
• Rapid increase of sphinganine (sometimes sphingosine).
• Increase of sphinganine degradation products like sphinganine 1-phosphate.
• Decrease of complex sphingolipids.
Free sphingoid bases are toxic to most cells by affecting cell proliferation and inducing apoptosis or necrotic cell death (Riley et al. 1996; Stevens et al. 1990). The accumulation of sphinganine is associated with hepato- and nephrotoxic effects (Riley et al. 1994). Complex sphingolipids are important for cell growth regulation and also cell-cell interactions. The accumulation of free sphingoid bases in the serum and urine are a useful biomarker for the exposure of fumonisins (Riley et al. 1993).
In humans, fumonisins have been associated with esophageal cancer. In certain regions (South Africa, China, Italy) an increasing risk of developing human oesophageal cancer with increasing consumption of maize and maize-based products like polenta has been recorded (Chu and Li 1994; Marasas et al. 1988; Franceschi et al. 1990). In these regions, relative high fumonisin concentrations were detected (see also Table 1). According to IARC (International Agency for Research on Cancer) fumonisins are classified as possible human carcinogens.
In the case of animals, horses are the most sensitive species to fumonisin toxicity. Fumonisins cause a disease syndrome called equine leukoencephalomalacia (ELEM) or “hole-in-the-head-disease” which affects the central nervous system followed by lesions in the brain (see Figure 3). Equids are mainly affected by ELEM. The first signs are reduced feed intake, lethargy followed by convulsions until death. Firstly Marasas et al. (1988) induced ELEM experimentally in horses with pure FB1 by intravenous injection (0.125 mg FB1/kg body weight; seven times within 9 days).
In pigs several studies indicate that fumonisins can cause porcine pulmonary edema (PPE). The first sign of fumonisin contamination is often a decreased feed intake. Within 4 – 7 days of ingesting contaminated feed, pigs show respiratory disorders followed by death due to acute pulmonary edema. These effects were also observed by feeding pure FB1 or with F. verticillioides cultures contaminated maize (Marasas, 2001).
Whereas ELEM and PPE appear to be species specific, numerous experiments show that fumonisins cause hepato- and nephrotoxic effects in all animal species. Fumonisins are associated with disturbances of the immune system, too (Voss et al. 2007).
The toxicity of hydrolyzed FB1 (HFB1) is not clarified as there are many controversial studies about its effects on the organism. It is possibly also hepato- and nephrotoxic. Through nixtamalization, a traditional production step for the preparation of tortillas in which corn is heated in calciumhydroxyd before further processing, fumonisins are changed into their hydrolyzed form (Sydenham et al. 1995). In a trial rats were fed with alkaline treated F. verticillioides cultures (51 ppm HFB1) and in comparison with untreated feed (91 ppm FB1). The ratio of sphinganine and sphingosine in the body liquids of the rats which were fed with alkali treated feed was considerably increased but still lower than in the group fed with untreated cultures (Voss et al. 1996; Voss et al. 1998). Additionally, Merrill et al. documented that the inhibition of the sphingoidbases by HFB1 is about a factor 10 less reduced than for FB1 (Merrill et al. 2001).
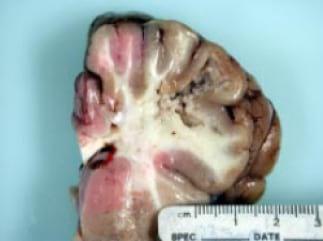
Figure 3: Effect of ELEM in the brain of horses
Source:http://www.vet.ksu.edu/features/VetQuarterly/KVQSum03.pdf; Accessed: 2008-01-04
Counteracting
Prevention measurements describe all the steps to counteract mycotoxins during the growth of the grain as well as during harvesting or storage. On the field all management practices which maximize plant performance and reduce plant stress can substantially decrease mycotoxin contamination. This includes pre-harvest practices like fertilization, proper crop rotation, avoidance of pests, optimal crop density and high quality seeds. In addition, appropriate harvest time as well optimal storage conditions like temperature or humidity control are important (Jouany, 2007). All these prevention measures can only reduce but not eliminate the risk of mycotoxin contamination. Therefore successful detoxification procedures after harvest are essential. They can be classified divided into three categories: physical, chemical and biological methods.
The efficacy of physical treatments depends on the level of contamination and the distribution of the mycotoxins in the grain. Additionally, the results obtained are often uncertain and associated with high losses. Various chemicals (bases, oxidizing agents, different gases etc) have been tested for their ability to detoxify mycotoxins but only a limited number of them are shown to be effective against them without reducing nutritive value, palatability of the feed or producing toxic by-products. For achieving adequate decontamination results several parameters like reaction time, temperature and moisture have to be monitored. Due to their uncertain and uneconomic results, the practical application of physical and chemical treatments is very limited.
Adsorption
Adsorbent agents are added to the feed and bind mycotoxins during digestion in the gastrointestinal tract resulting in a reduction of toxin bioavailability. Adsorption of mycotoxins requires molecule polarity and also a suitable position of the functional groups. Due to this fact only a few mycotoxins can be adsorbed efficiently without affecting essential feed ingredients. This method is especially used to counteract aflatoxins, however in the case of fumonisins it’s only of limited success. In a study, different adsorbents were tested for their potential to bind FB1. An effective adsorption of FB1 was described with activated charcoal and cholestyramin in vitro. However, activated charcoal is a very unspecific adsorbent and binds valuable nutrients as well, therefore these results could only be confirmed for cholestyramin in vivo (Solfrizzo et al., 2001; Avantaggiato et al. 2005). Avantaggiato et al. (2005) from the Institute of Science of Food Production (ISPA) and the National Research Council (CNR), Bari, Italy, found out that among the commercially available feed additives Mycofix® Plus from Biomin GmbH showed good results with adsorption rates of 100% and 77% of 2 μg/ml and 20 μg/ml FB1, respectively.
Biotransformation
Fumonisins are natural toxins, and therefore they are biodegradable by natural metabolic pathways. Compared to adsorption of mycotoxins by clay, microbial biodegradation has the advantages of being highly specific and irreversible. Several microbial strains which are capable of fumonisin biodegradation were previously isolated, and the genes encoding fumonisin detoxification enzymes were identified (Blackwell et al. 1999: Duvick et al. 1998a; Duvick et al 1998b). Biomin GmbH has a long standing expertise in the application of specific microbes for mycotoxin biodegradation in the gastrointestinal tract of animals (Schatzmayr et al. 2006). Recently, Biomin GmbH scientists isolated and characterized new fumonisin-metabolizing bacterial strains (Schatzmayr et al. 2007). Some of these isolates were found to be active in the gastrointestinal tract of animals. One of the strains with the highest technological potential belongs to the family of the Sphingomonadaceae and was called MTA144. It degrades fumonisins by first cleaving off tricarballylic acid side chains, and subsequently catabolising the rest of the molecule into non-toxic products. Development of a novel feed additive for fumonisin detoxification based on this strain - whose efficacy was proven in vitro - is in progress. Nevertheless in vivo trials are necessary to prove its efficacy in the animals.
Conclusion
Although extensive efforts on preventive actions during crop growth, harvesting and storage are taken, there is still a potential risk of mycotoxin contamination. As a result of their different structures mycotoxins can cause various toxic effects in animals. Therefore, there cannot be only one effective strategy against mycotoxins. Whilst adsorption is very efficient for aflatoxins, this method is quite limited for other mycotoxins. Only a feed additive which combines adsorption and biotransformation will lead to success.
References:
ApSimon, J. W. (2001) Structure, synthesis, and biosynthesis of fumonisin B1 and related compounds. Environmental Health Perspectives 109, 245-249.
Avantaggiato, G., Solfrizzo, M. and Visconti, A. (2005) Recent advances on the use of adsorbent materials for detoxification of Fusarium mycotoxins. Food Additives & Contaminations 22, 379-388.
Berg, J. M., Tymoczko, J. L. and Stryer, L. (2003) Biochemie. Spektrum Akademischer Verlag Heidelberg, Berlin.
Binder, E. M., Tan, L. M., Chin, L. J., Handl, J. and Richard, J. (2007) Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Animal Feed Science and Technology 137, 265-282.
Bottalico, A, Logrieco, A, Ritieni, A, Moretti, A, Randazzo, G. and Corda P. (1995). Beauvericin and fumonisin B1 in preharvest Fusarium moniliforme maize ear rot in Sardinia. Food Additives & Contaminations 12, 599-607.
Blackwell, B. A., Gilliam, J. T., Savard, M. E., David Miller, J. and Duvick, J. P. (1999). Oxidative deamination of hydrolyzed fumonisin B(1) (AP(1)) by cultures of Exophiala spinifera. Natural Toxins 7, 31-38.
CAST Report (2003) Mycotoxins: risks in plant, animal, and human systems. In: Richard, J.L. and Payne,G.A. (Eds.), Council for Agricultural Science and Technology Task Force Report No. 139, Ames, Iowa, USA.
Chin, L.-J. and Tan, L.-M. (2006) Occurrence of mycotoxins in Asian feed samples. Asian Pork Magazine June/July, 20-25.
Chu, F.S. and Li, Y. (1994) Simultaneous occurrence of fumonisin B1 and other mycotoxins in moldy corn collected from the People's Republic of China in regions with high incidences of esophageal cancer. Applied and Environmental Microbiology 60, 847-852.
Duvick, J., Rood, T. and Wang, X. (1998a). Fumonisin Detoxification Enzymes. US Patent 5,716,820.
Duvick, J., Rood, T., Maddox, J. and Wang, X. (1998b). Fumonisin Detoxification Composition and Methods. US Patent 5,792,931
Fazekas, B., Bajmocy, E., Glavits, R., Fenyvesi, A. and Tanyi, J. (1998). Fumonisin B1 contamination of maize and experimental acute fumonisin toxicosis in pigs. Journal of Veterinary Medicine. Series B 45, 171-181.
Franceschi, S., Bidoli, E., Baron, A. E. and La, V. C. (1990) Maize and risk of cancers of the oral cavity, pharynx, and esophagus in northeastern Italy. Journal of the National Cancer Institute 82, 1407-1411.
Gelderblom, W. C., Jaskiewicz, K., Marasas, W. F., Thiel, P. G., Horak, R. M., Vleggaar, R. and Kriek, N. P. (1988) Fumonisins--novel mycotoxins with cancerpromoting activity produced by Fusarium moniliforme. Applied and Environmental Microbiology 54, 1806-1811.
Gelderblom, W. C., Kriek, N. P., Marasas, W. F. and Thiel, P. G. (1991) Toxicity and carcinogenicity of the Fusarium moniliforme metabolite, fumonisin B1, in rats. Carcinogenesis 12, 1247-1251.
Jouany, J. P. (2007) Methods for preventing, decontaminating and minimizing the toxicity of mycotoxins in feeds. Animal Feed Science and Technology 137, 342–362.
Krska, R., Welzig, E. and Boudra, H. 2007. Analysis of Fusarium toxins in feed. Animal Feed Science and Technology 137, 241-264.
Marasas, W. F. (2001) Discovery and occurrence of the fumonisins: a historical perspective. Environmental Health Perspective 109(2), 239-243.
Marasas, W. F. (1996) Fumonisins: history, world-wide occurrence and impact. Advances in Experimental Medicine and Biology 392, 1-17.
Marasas, W. F., Kellerman, T. S., Gelderblom, W. C., Coetzer, J. A., Thiel, P. G. and van der Lugt, J. J. (1988) Leukoencephalomalacia in a horse induced by fumonisin B1 isolated from Fusarium moniliforme. The Onderstepoort Journal of Veterinary Research 55, 197-203.
Marin, S., Magan, N., Belli, N., Ramos, A.J., Canela, R. and Sanchis, V. (1999) Twodimensional profiles of fumonisin B1 production by Fusarium moniliforme and Fusarium proliferatum in relation to environmental factors and potential for modelling toxin formation in maize grain. International Journal of Food Microbiology 51, 159-167.
Merrill, A. H., Jr., Sullards, M. C., Wang, E., Voss, K. A. and Riley, R. T. (2001). Sphingolipid metabolism: roles in signal transduction and disruption by fumonisins. Environmental Health Perspective 109(2), 283-289.
Miller, J. D. (1995) Fungi and mycotoxins in grain: Implications for stored product research. Journal of Stored Products Research 31, 1-16.
Rheeder, J. P. and Marasas, W. F. (1998) Fusarium species from plant debris associated with soils from maize production areas in the Transkei region of South Africa. Mycopathologia 143, 113-119.
Rheeder, J. P., Marasas, W. F. and Vismer, H. F. (2002) Production of fumonisin analogs by Fusarium species. Applied and Environmental Microbiology 68, 2101-2105.
Riley, R. T., An, N. H., Showker, J. L., Yoo, H. S., Norred, W. P., Chamberlain, W. J., Wang, E., Merrill, A. H., Jr., Motelin, G. and Beasley,V.R. (1993) Alteration of tissue and serum sphinganine to sphingosine ratio: an early biomarker of exposure to fumonisin-containing feeds in pigs. Toxicology and Applied Pharmacology 118, 105-112.
Riley, R. T., Hinton, D. M., Chamberlain, W. J., Bacon, C. W., Wang, E., Merrill, A. H., Jr. and Voss, K. A. (1994) Dietary fumonisin B1 induces disruption of sphingolipid metabolism in Sprague-Dawley rats: a new mechanism of nephrotoxicity. The Journal of Nutrition 124, 594-603.
Riley, R. T., Wang, E., Schroeder, J. J., Smith, E. R., Plattner, R. D., Abbas, H., Yoo, H. S. and Merrill, A. H., Jr. (1996) Evidence for disruption of sphingolipid metabolism as a contributing factor in the toxicity and carcinogenicity of fumonisins. Natural Toxins 4, 3-15.
Ross, P. F., Rice, L. G., Osweiler, G. D., Nelson, P. E., Richard, J. L. and Wilson, T. M. (1992) A review and update of animal toxicoses associated with fumonisincontaminated feeds and production of fumonisins by Fusarium isolates. Mycopathologia 117, 109-114.
Roth, L., Frank, H. and Kormann, K. (1990) Schimmelpilze und Mycotoxine. In Giftpilze Pilzgifte. Nikol, Hamburg. pp. 18-29.
Schatzmayr, G., Zehner, F., Taubel, M., Schatzmayr, D., Klimitsch, A., Loibner, A. P. and Binder, E. M. (2006). Microbiologicals for deactivating mycotoxins. Molecular Nutrition & Food Research 50, 543-551.
Schatzmayr, G., Täubel, M., Vekiru, E. and Binder, E. M. (2007). Micro-organism for decontaminating fumonisins and its use,method for decontaminating fumonisins and feed additives containing said micro-organism. PCT patent application WO2006053357
Seo, J. A. and Lee, Y. W. (1999). Natural occurrence of the C series of fumonisins in moldy corn. Applied and Environmental Microbiology 65, 1331-1334.
Solfrizzo, M., Carratu, M. R., Avantaggiato, G., Galvano, F., Pietri, A., Visconti, A. (2001) Ineffectiveness of activated carbon in reducing the alteration of sphingolipid metabolism in rats exposed to fumonisin-contaminated diets. Food and Chemical Toxicology 39, 507-511.
Stevens, V. L., Nimkar, S., Jamison, W. C., Liotta, D. C. and Merrill, A. H., Jr. (1990) Characteristics of the growth inhibition and cytotoxicity of long-chain (sphingoid) bases for Chinese hamster ovary cells: evidence for an involvement of protein kinase C. Biochimica et Biophysica Acta 1051, 37-45.
Sydenham, E. W., Stockenstrom, S., Thiel, P. G., Shephard, G. S., Koch, K. R. and Marasas, W. F. O. (1995) Potential of Alkaline-Hydrolysis for the Removal of Fumonisins from Contaminated Corn. Journal of Agricultural and Food Chemistry 43, 1198-1201.
Tan, L.M. (2007) Occurrence of mycotoxins in Asia. Feed Mix 15(4), 32-33.
Voss, K. A., Bacon, C. W., Meredith, F. I., Norred, W. P. (1996). Comparative subchronic toxicity studies of nixtamalized and water-extracted Fusarium moniliforme culture material. Food and Chemical Toxicology 34, 623-632.
Voss, K. A., Riley, R. T., Bacon, C. W., Meredith, F. I. and Norred, W. P. (1998). Toxicity and sphinganine levels are correlated in rats fed fumonisin B1 (FB1) or hydrolyzed FB1. Environmental Toxicology and Pharmacology 5, 101-104.
Voss, K. A., Smith, G. W. and Haschek, W. M. (2007). Fumonisins: Toxicokinetics, mechanism of action and toxicity. Animal Feed Science and Technology 137, 299–325.
Wang, E., Norred, W. P., Bacon, C. W., Riley, R. T. and Merrill ,A .H., Jr. (1991) Inhibition of sphingolipid biosynthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme. The Journal of Biological Chemistry 266, 14486-14490.
Wilson, T.M., Ross, P. F., Rice, L. G., Osweiler, G. D., Nelson, H. A., Owens, D. L., Plattner, R. D., Reggiardo, C., Noon, T. H. and Pickrell, J. W. 1990. Fumonisin B1 levels associated with an epizootic of equine leukoencephalomalacia. Journal of Veterinary Diagnostic Investigation 2, 213-216.
Related topics:
Authors:
DSM-Firmenich
Recommend
Comment
Share
13 de agosto de 2008
The much work information about involvement of fumonisins in causing Degnala disease in buffalo and its pathogenecity and immunologic properties need to be evaluated.
Recommend
Reply

Would you like to discuss another topic? Create a new post to engage with experts in the community.







.jpg&w=3840&q=75)