INTRODUCTION
Aflatoxins (AF) are toxic metabolites produced by fungi, such as Aspergillus flavus and Aspergillus parasiticus, and are considered to be the most harmful mycotoxins as they are highly toxic to animals and humans (Kaya, 1989; Hoerr, 2003). There are six different kinds of aflatoxins: B1, B2, G1, G2, M1, M2. Aflatoxin B1 (AFB1) is accepted as the most harmful, as well as most common metabolite in feeds and foods (Hatch, 1988). Aflatoxicosis in quails has been thoroughly investigated by many scientists, and was shown to be as economically important for quail production as for that of other poultry (Parlat et al., 2001; Oliveira et al., 2002; Oguz & Parlat, 2004; Sehu et al., 2005; Cengiz et al., 2008). Aflatoxins impair performance parameters, such as feed conversion ratio, body weight gain, egg production, and growth rate in all poultry species (Erdeger, 2002). Similarly, it has been reported that AF have adverse effects on the growth performance and health of quails, and as well as carcinogenic and mutagenic effects (Parlat et al., 2001).
When orally ingested with water and feed, AF are absorbed in the digestive tract and are bound to serum albumin. Circulating AF are largely metabolized in the liver. Part of the AF bind to macromolecules, such as endoplasmic steroids and enzymes in the hepatocytes, whereas the remaining part is converted into fat – and water-soluble metabolites. AF itself is actually harmless, but its metabolization in the liver through cytochrome P-450, produces epoxide derivatives. After this stage, AF acquires toxic properties and plays a role in the inhibition of DNA, RNA, and protein synthesis in liver. In addition, its binding to several macromolecules causes cytotoxic, carcinogenic and teratogenic effects (Abdel-Wahhab & Aly, 2005). In addition, acute and chronic poisoning, as well as mutagenic and immunosuppressive effects have been reported (Kiran et al., 1998; Sur & Celik, 2003).
Duck and turkeys are the most sensitive poultry species to aflatoxicosis, quails are moderately susceptible, whereas chickens are considered the most resistant. It has been reported that pheasants, geese, and chickens are more resistant than ducks and turkeys. Bobwite quails are more susceptible than Japanese quails (Ruff et al., 1992). The most obvious macroscopic findings related to acute and chronic aflatoxicosis are observed in the liver. Lesions can also be observed in other organs, such as the kidneys, spleen and bursa of Fabricius (Diaz et al., 2008).
As in other poultry, AF can cause depression, anorexia, icterus, hemorrhages, and death in quails (Oliveira et al., 2002). The most obvious macroscopic findings are observed in the liver in acute and chronic aflatoxicosis. In addition, lesions are found in other organs such as the kidney, spleen, and bursa of Fabricius (Sawhney et al., 1973; Ortatatli et al., 2005). At gross examination, the liver is pale and enlarged. The main histopathological findings are oil vacuoles in the hepatocytes, hydropic degeneration, necrosis, and bile duct proliferation (Ortatatli & Oguz, 2001). The kidney and spleen may be enlarged, and their surfaces may present petechial hemorrhages (Bilgic & Yesildere, 1992). Tubular degeneration and capillary hyperemia are commonly observed in the kidneys, and lymphoid-cell depletion and necrosis in the spleen. The bursa of Fabricius may present lymphoid-cell depletion and intrafollicular cysts (Ortatatli et al., 2002).
Glucomannans (GM) are extracted from the cell wall of the live yeast Saccharomyces cerevisiae, and were used to prevent the absorption of mycotoxins in the early 1990s (Stanley et al., 1993). It was demonstrated that GM present a strong capacity to bind AF in vivo and in vitro when added to poultry diets (Bintvihok et al., 2002; Karaman et al., 2005; Oguz, 2011; Azizpour & Moghadam, 2015). The positive effect of GM on oxidative stress parameters was also shown in quails (Atalay, 2010).
The present study investigated if the dietary addition of different doses of GM were effective for the protection of Japanese quails against aflatoxicosis. For this purpose, relative organ weights (liver, kidney, spleen, bursa of Fabricius and thymus) were calculated, and pathological changes in the organs were compared.
MATERIALS AND METHODS
Birds and treatments
Sixty one-day old male Japanese quails (Coturnix coturnix japonica) were evaluated. The quails were obtained from Selçuk University, Faculty of Agriculture, Poultry Unit. The quails were housed in electrically heated cages, at a density of 30 birds per cage, and provided with continuous lighting. A commercial basal diet (65% corn, 32% soybean meal, 3% vitamin-mineral premix), supplemented with amino acids, minerals, and vitamins at the levels recommended by the National Research Council (NRC, 1994), was fed. Before being supplied, the basal diet was proven to be clear from any detectable levels of residual AF (Howell & Taylor, 1981) at a detection limit of 1µg/kg feed, using thin-layer chromatography (TLC) at 95% recovery rate. This basal diet was contaminated or not with aflatoxin and supplemented with a glucomannan at 1 or 2 g/kg of diet, according to the treatments: control (C), basal diet; aflatoxin (A), 2mg aflatoxin/kg basal diet; glucomannan (GM), 1g glucomannan/kg basal diet; two-fold dose of glucomannan (2GM), 2g glucomannan/kg basal diet;aflatoxin + glucomannan (A+GM), basal diet containing 2mg aflatoxin/kg plus 1g glucomannan/kg; and aflatoxin + two-fold dose of glucomannan (A+2GM), basal diet containing 2mg aflatoxin/kg plus 2g glucomannan/kg. The Japanese quails were equally divided into the six treatments with 10 birds each, and were fed the experimental diets from one to 21 days of age.
Aflatoxin and glucomannan analyses
The AF content in rice powder was analyzed and measured on thin layer chromatography-fluorometric densitometer (TLC) (Camag II, Basel, Switzerland). The AF was produced from a culture of Aspergillus parasiticus NRRL 2999 (USDA, Agricultural Research Service, Peoria, IL) via rice fermentation by the method of Shotwell et al. (1966), with minor modifications by Demet et al. (1995) and Oguz (1997). Briefly, 100 g of sterile polished rice were inoculated with 1 mL of resuspended spores (1.5 x 106 spores/mL) of Aspergillus parasiticus NRRL 2999, placed an incubator at 28°C, and fermented for five days. AF was extracted as follows. Briefly, 10 g of fermented rice powder were accurately weighed and dispersed in 100 mL of distilled water and fixed for five minutes. Then, 100 mL of chloroform were added to the solution and blended for 15 minutes at 3000 rpm in a centrifuge. The chloroform phase was taken into a separating funnel already containing 15 g of anhydrous sodium sulfate. The collected chloroform evaporated to dryness in a rotary evaporator. Dried sample extracts were individually (2, 5, 10 µL) applied on the TLC plates, which were developed in an unlined tank containing 20 mL of chloroform:xylene:acetone (7:2:1; v/v) and observed under UV light (365 nm wavelengths and 425 emission).The AF in the rice powder consisted of 82.3% AFB1, 2.06% AFB2, 7.68% AFG1, and 7.96% AFG2 based on total AF in the ground rice powder (detection limit of aflatoxin: 1µg aflatoxin/kg rice powder. Recovery of the extraction method: 92%). The rice powder was incorporated into the basal diet in order to provide the desired amount of 2 mg AF/kg feed.
Esterified glucomannan was used as binding agent of AF.
Pathological Examination
All quails in each group on day 21 of the study were weighed, euthanized by decapitation, and necropsied. The liver, kidneys, spleen, thymus and bursa of Fabricius were collected and weighed on a precision scale to calculate relative organ weight as a percentage of live weight.
The collected organs were fixed in 10% formalin solution, dehydrated in graded alcohol series, cleared in xylene, and embedded in paraffin blocks. Tissues were cut in 5-µm thick sections using a microtome, mounted on slides, stained with hematoxylin and eosin (Luna, 1968), and examined under a light microscope.
In histopathological examination, liver degenerative changes were scored according to the method described by Ortatatli et al. (2005). Mild hepatocellular swellings due to hydropic and fatty degeneration only in centrilobular areas were scored as 1 (Mild);evident hepatocellular swelling in the centrilobular and the intermediate areas of lobules, were scored as 2 (Intermediate); and severe hepatocellular swellings extending to the whole lobules were scored as 3 (Severe). Lymphoid depletion in the bursa of Fabricius and spleen, cortical atrophy in the thymus, and tubular degeneration in the kidney were evaluated by histopathology.
Statistical Analysis
The Kruskal-Wallis test was applied to compare the gross and histopathological findings among treatments. Relative organ weight differences were compared by Duncan’s multiple range test (IBM® SPSS® Statistics Version 22). Statements of statistical significance are based on a p value of <0.05.
RESULTS
Pathological findings of liver, spleen, kidney, thymus and bursa of Fabricius are summarized in Table 1.
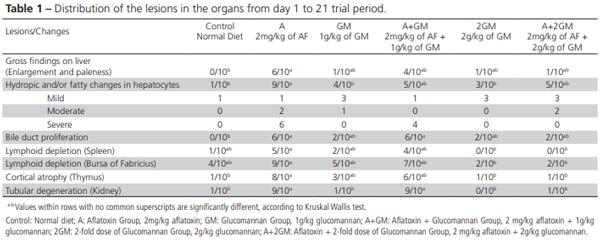
Liver gross lesions were observed in the livers of six quails of group A, in four of the group A+GM and only one bird of the group A+2GM (Figure 1). The highest frequency of fatty and hydropic liver degeneration (9/10 birds) was determined in group A, and the lowest in the GM and 2GM groups (3 and 4/10 birds, respectively), while groups A+GM and A+2GM presented intermediate frequencies (5/10 birds). Relative to severity, only groups A and A+GM presented severe lesions (6 and 4 birds, respectively). Moderate lesions were observed in 3 A+2GM birds, 2 A birds, and one GM bird, but not in the other groups. Mild lesions were detected in 1/10 bird of the Control, A, and A+GM groups, and 3/10 birds of the GM, 2GM, and A+GM groups.
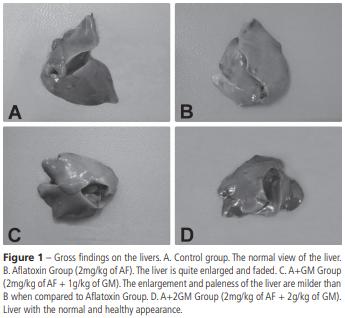
A higher frequency of bile duct proliferation and periportal fibrosis was detected in Groups A and A+GM (6 birds) compared with the groups fed GM (2 birds) (Figure 2).
In the spleen, lymphoid cell depletion was observed in 5/10 birds of group A, 4/10birds of group A+GM, and 1/10 bird of group GM. No lymphoid cell depletion was detected in groups 2GM and A+2GM.
In the bursa, 9/10 birds of group A presented the highest central lymphoid cell depletion, as well as intrafollicular cysts. Groups A+GM and GM presented intermediate frequencies (7/10 and 5/10 birds, respectively), whereas no depletion was detected in birds of groups 2GM and A+2GM.
Group A presented the highest number of birds (8/10) with thymus cortical atrophy, followed by groups A+GM and GM with intermediate frequencies (6/10 and 3/10, respectively), and groups 2GM and A+2GM, the lowest, with only 1/10 birds each.
No kidney necrosis and hemorrhages were found. Degenerative changes in renal tubular epithelia were observed in a higher number of birds of groups A and A+GM (9/10 each) compared with the other groups (1/10 in GM and A+2GM and none in the 2GM group), as shown in Table 1.
The relative organ weights of liver, kidney, spleen, bursa of Fabricius, and thymus are presented in Table 2.There were no differences in the relative weights of the kidneys and spleen among treatments. Birds of group A presented the heaviest livers and the A+GM and 2GM birds, the lightest, whereas the relative liver weights of the GM and the A+GM birds had intermediate values. The bursa of A+GM birds was lighter than those of the 2GM birds, whereas the other groups presented intermediate values. The highest and the lowest relative thymus weight were observed in group A+2GM and A+GM, respectively, whereas the remaining groups weren’t statistically different from each other.
DISCUSSION
Macroscopic and microscopic examinations are very effective methods to demonstrate the pathological findings of aflatoxicosis (Bintvihok et al., 2002). In particular, it was reported that the liver, kidney, spleen, thymus, and bursa of Fabricius are the target organs of aflatoxicosis (Ortatatli et al., 2005). As mentioned in previous studies, aflatoxicosis histopathological findings are directly proportional to the exposure time and intensity of the toxin (Magnoli et al., 2012). In 21- day trial period of the present study, the toxic effects of AF and the protective effects of GM were histologically demonstrated.
Liver paleness and hepatomegaly are important indicators of aflatoxicosis (Hoerr, 2003; Ortatatli et al., 2005). While no gross changes were observed in the Control, GM and 2GM groups, the livers of the birds of the A and A+GM groups were very swollen and pale. In contrast, the severity of liver lesions was mild in the A+2GM group, whereas 6/10 and 4/10 birds of the A and A+GM groups presented severe lesions. The difference between these groups (A and A+2GM) was statistically significant. The macroscopic liver changes observed in the present study are consistent with previously reported studies (Sawhney et al., 1973; Ortatatli & Oguz, 2001; Oliveira et al., 2002;Ortatatli et al., 2005; Citil et al., 2007; Magnoli et al., 2012; Ibrahim, 2013).
The most common finding in aflatoxicosis is fatty and hydropic degeneration of the liver, as observed by microscopic examination (Sawhney et al., 1973; Bryden & Cumming, 1980; Ortatatli & Oguz, 2001; Oliveira et al., 2002; Karaman et al., 2005; Ortatatli et al., 2005; Attia et al., 2016). No severe lesions were observed in the Control, GM, 2GM, and A+2GM birds. Severe hydropic and fatty degeneration were present in the livers of group A. In addition, marked diffuse fatty changes, bile duct proliferation, and periportal fibrosis were detected in the portal areas of those birds. In a similar study in broiler chickens, broilers were fed 2 mg aflatoxins/kg diet+0.5g or 1 g glucomannan/kg diet and reported very mild liver lesions with the addition of glucomannan (Karaman et al., 2005). In the present study, the severity of the hydropic and fatty degeneration was reduced when broilers fed the AF-contaminated diet received 2g of GM compared with 1g.
Lymphoid organs, such as the spleen, bursa of Fabricius, and thymus are primarily affected by aflatoxicosis (Ortatatli et al., 2005). Pathological changes in the lymphoid organs caused by aflatoxicosis were microscopically shown in the present study. Lymphoid cell depletion in bursa of Fabricius, thymus and spleen were more frequently observed in groups A and A+GM. Necrosis of the germinal centers of the lymphoid follicles and rarely intrafollicular cysts were found in these groups. Cortical atrophy of thymus and necrosis and spleen lymphoid tissue depletion were evident. The highest frequency of birds with lymphoid tissue depletion both in the spleen and the bursa were determined in group A, whereas the lowest in groups 2GM and A+2GM. The lesions of group A+2GM were mild and found in a limited number of birds. The number of birds with lymphoid lesions in the control, GM and 2GM groups was not statistically different from the A and the A+2GM groups.
Due to the short duration of this study, only degenerative changes were observed in the kidneys, and not necrosis and intertubular hemorrhages commonly observed with the chronic intoxication with aflatoxin (Bilgic & Yesildere, 1992; Valchev et al., 2014).
Kubena et al. (1990) observed that changes in liver weight are a more sensitive indicator of aflatoxicosis compared with the weight of other organs, such as kidneys, spleen, and pancreas. In our study, relative liver weight was higher in group A compared with the Control, GM and A+2GM groups, whereas the groups 2GM and A+GM presented intermediate values. The heavier livers of the birds fed only aflatoxin may be due to the histopathological fatty changes in the hepatocytes, as previously reported in other studies (Ortatatli & Oguz, 2001; Ortatatli et al., 2005). Raju & Devegowda (2000) reported that an esterified glucomannan reduced liver weight, but did not affect kidney weight, as also observed in the present study in the birds fed aflatoxin and 2 g GM/kg of diet (A+2GM).
The relative weights of the bursa of Fabricius and thymus were affected by the dietary treatments. In particular, the birds fed only AF presented significant atrophy of these organs compared with the controls, probably due to lymphoid tissue depletion and necrosis of the parenchyma of these organs (Ortatatli & Oguz, 2001; Girish & Devegowda, 2006). However, the addition of 2 g glucomannan/kg to the AF-containing diet (A+2GM group) statistically increased the relative weights of the bursa and thymus in comparison with the supplementation of 1 g glucomannan/kg of the AF-containing diet.
Therefore, the addition of 2 g of glucomannan per kg of an aflatoxin-contaminated diet may protect Japanese quails from aflatoxicosis, as demonstrated by the alleviation of gross and pathological changes in the liver and lymphoid organs.
This article was originally published in Revista Brasileira de Ciencia Avicola, vol.19, no.3, Campinas July/Sept. 2017. http://dx.doi.org/10.1590/1806-9061-2016-0349. This is an Open Access article distributed under the terms of the Creative Commons Attribution License. REFERENCES
Azizpour A, Moghadam N. Effects of yeast glucomannan and sodium bentonite on the toxicity of aflatoxin in broilers. Brazilian Journal of Poultry Science 2015;17:7-13.
Atalay B. The effects of dietary esterified glucomannan on lipid peroxidation and some antioxidant system parameters during experimental aflatoxicosis in japanese quails. Dicle Üniversitesi Veteriner Fakültesi Dergisi 2010;2:29-33.
Attia YA, Abd Al-Hamid AE, Allakany HF, Al-Harthi MA, Mohamed NA. Necessity of continuing of supplementation of non-nutritive feed additive during days 21–42 of age following 3 weeks of feeding aflatoxin to broiler chickens, Jornal of Applied Animal Research 2016;44:87-98.
Abdel-Wahhab MA, Aly SE. Antioxidant property of nigella sativa (black cumin) and syzygium aromaticum (clove) in rats during aflatoxicosis, Journalof Applied Toxicology 2005;25:218-223.
Bilgic HN, Yesildere T. Renal lesions on experimental aflatoxicosis in chickens. Istanbul Üniversitesi Veteriner Fakültesi Dergisi 1992;18:102-108.
Bintvihok A, Banlunara W, Kaewamatawong T. Aflatoxin detoxification by esterified glucomannan in ducklings. Journal of Health Research 2002;16:135-148.
Bryden WL, Cumming RB. Observations on the liver of the chicken following aflatoxin B1 ingestion. Avian Pathology 1980;9:551-556.
Cengiz Ö, Cakir S, Sehu A, Essiz D, Ergün E, Ergün L, et al. Effects of Moldstop® on aflatoxicosis in quails. Journal of Veterinary Research 2008;52:561 – 564.
Citil M, Karapehlivan M, Tuzcu M, Dogan A, Uzlu E, Atakisi E, et al. Effect of l-carnitine supplementation on biochemical, haematological andpathological parameters of quails (coturnix coturnix japonica) during chronic aflatoxicosis. Kafkas Üniversitesi Veteriner Fakültesi Dergisi 2007;13:75-85.
Demet O, Oguz H, Celik I, Nizamlioglu F. Pirinçte aflatoksin üretilmesi. Eurasian Journal of Veterinary Science 1995;11:19-23.
Diaz GJ, Calabrese E, Blain R. Aflatoxicosis in chickens (gallus gallus). an example of hormesis. Poultry Science 2008;87(4):727-732.
Eraslan G, Liman BC, Guclu BK, Atasever A, Koc AN, Beyaz L. Evaluation of aflatoxin toxicity in japanese quails given various doses of hydrated sodium calcium aluminosilicate. Bulletin of the Veterinary Institute in Pulawy 2004;48:511-517.
Erdeger J. Aflatoksikozis. Kanatli hayvan hastaliklari. Ankara: Medisan Press; 2002.
Girish CK, Devegowda G. Efficacy of glucomannan-containing yeast product (mycosorb) and hydrated sodium calcium aluminosilicate in preventing the individual and combined toxicity of aflatoxin and t-2 toxin in commercial broilers. Asian-Australasian Journal of Animal Sciences 2006;19:877 – 883.
Hatch RC. Veterinary pharmacology and therapeutics. 6th ed. Ames: Iowa State University Press; 1988.
Hoerr JF. Mycotoxicoses. In: Calnek BW, et al. Diseases of poultry. 11th ed. Ames: Iowa State University Press; 2003.
Howell MV, Taylor PW. Determination of aflatoxins, ochratoxin a, and zearalenone in mixed feeds, with detection by thin layer chromatography or high performance liquid chromatography. Journal - Association of Official Analytical Chemists 1981;64:1356-1363.
Ibrahim QQ. Histopathological study of quails liver experimentally induced by aflatoxin. Basrah Journal of Veterinary Research 2013;12:116-127.
Karaman M, Basmacioglu H, Ortatatli M, Oguz H. Evaluation of the detoxifying effect of yeast glucomannan on aflatoxicosis in broilers as assessed by gross examination and histopathology. British Poultry Science 2005;46:394-400.
Kaya S. Yem ve besinlerdeki mikotoksinler: insan hayvan sagligi için önemleri. Ankara Universitesi Veteriner Fakültesi Dergisi 1989;36:226- 253.
Kiran MM, Demet O, Ortatatli M, Oguz H. The preventive effect of polyvinyl–polypyrrolidone on aflatoxicosis in broilers. Avian Pathology 1998;27:250–255.
Kubena LF, Harvey RB, Huff WE, Corrier DE, Phillips TD, Rottinghaus GE.Efficacy of hydrated sodium calcium aluminosilicate to reduce the toxicity of aflatoxin and t-2 toxin. Poultry Science 1990;69:1078-1086.
Luna LG. Processing of tissue. In: Luna LG. Manual of histology staining methods of the armed forces institute of pathology. 3rd ed. New York; 1968.
Magnoli AP, Monge MP, Nazar FN, Magnoli CE, Cavaglieri LR, Bagnis G, et al. Combined effects of aflatoxin B1 and corticosterone treatment on selected performance indices and liver histopathology in Japanese quail. Poultry Science 2012;91:354–361.
NRC - National Research Council. Nutrient requirements of poultry. 9th ed. Washington; 1994.
Oguz H. The preventive efficacy of polyvinylpolypyrrolidone (PVPP) alone and its combination with the other adsorbents into broiler feeds against aflatoxicosis [dissertation]. Konya (TR): University of Selcuk; 1997.
Oguz H, Parlat SS. Effects of dietary mannanoligosaccharide on performence of japanese quail affected by aflatoxicosis. South African Journal of Animal Science 2004;34:144–148.
Oguz H. A review from experimental trials on detoxification of aflatoxin in poultry feed. Eurasian Journal of Veterinary Science 2011;27:1-12.
Oliveira CAF, Rosmaninho JF, Butkeraitis P, Correa B, Reis A, Guerra JL, et al. Effect of low levels of dietary aflatoxin b1 on laying japanese quail. Poultry Science 2002;81:976–980.
Ortatatli M, Oguz H. Ameliorative effects of dietary clinoptilolite on pathological changes in broiler chickens during aflatoxicosis. Research in Veterinary Science 2001;71:59–66.
Ortatatli M, Ciftci MK, Tuzcu M, Kaya A. The effects of aflatoxin on the reproductive system of roosters. Research in Veterinary Science 2002;72:29–36.
Ortatatli M, Oguz H, Hatipoglu F, Karaman M. Evaluation of pathological changes in broilers during chronic aflatoxin (50 and 100 ppb) and clinoptilolite exposure. Research in Veterinary Science 2005;78:61–68.
Parlat SS, Ozcan M, Oguz H. Biological suppresion of aflatoxicosis in japanese quail (coturnix coturnix japonica) by dietary addition of yeast (saccharomyces cerevisae). Research in Veterinary Science 2001;71:207 – 211.
Raju MVLN, Devegowda G. Influence of esterified-glucomannan on performance and organ morphology, serum biochemistry and haematology in broilers exposed to individual and combined mycotoxicosis (aflatoxin, ochratoxin and T-2 toxin). British Poultry Science 2000;41:640-650.
Ruff MD, Huff WE, Wilkins GC. Characterization of the toxicity of the mycotoxins aflatoxin, ochratoxin, and t-2 toxin in game birds. III. bobwhite and japanese quail. Avian Diseases 1992;36:34-39.
Sawhney DS, Vadehra DV, Baker RC. Aflatoxicosis in the laying japanese quail (coturnix coturnix japonica). Poultry Science 1973;52:465-473.
Sehu A, Cakir S, Cengiz Ö, Essiz D. Mycotox® and aflatoxicosis in quails. British Poultry Science 2005;46:520-524.
Shotwell OL, Hesseltine CV, Stubblefield RD, Sorenson WG. Production of aflatoxin on rice. Journal of Applied Microbiology 1966;14:425–429.
Stanley VG, Ojo R, Woldensenbet S, Hutchinson DH. The use of Saccharomyces cerevisiae, to suppress the effect of aflatoxicosis in broiler chicks. Poultry Science 1993;72:1867-1872.
Sur E, Celik I. Effects of aflatoxin b1 on the development of bursa of fabricius and blood lymphocyte acid phosphatase of the chicken. British Poultry Science 2003;44:558–566.
Valchev I, Kanakov D, Hristov TS, Lazarov L, Binev R, Grozeva N, et al. Effects of experimental aflatoxicosis on renal function in broiler chickens. Bulgarian Journal of Veterinay Medicine 2014;17:302-313.