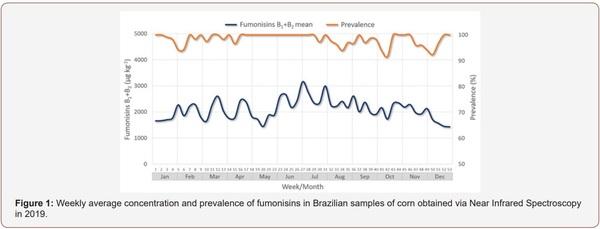
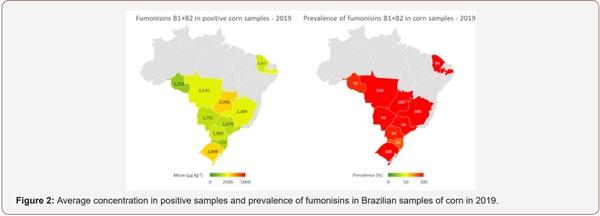
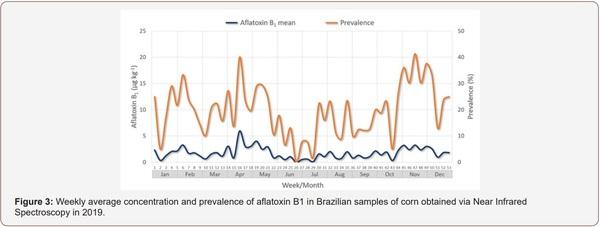
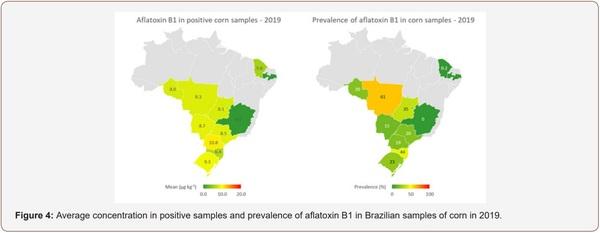
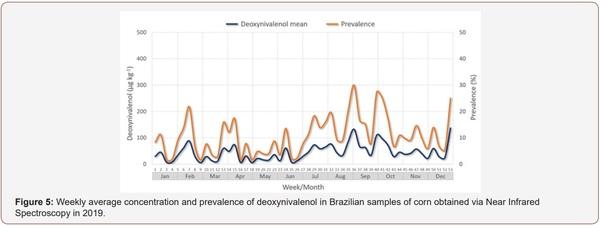
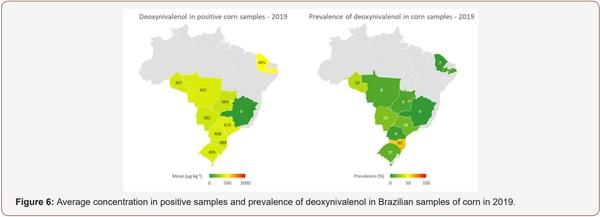
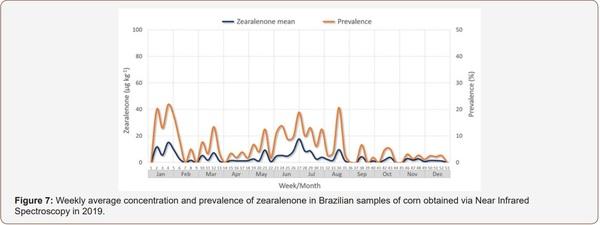
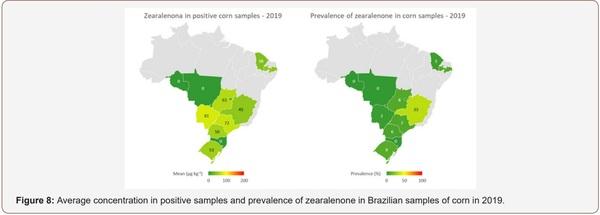
Survey of Mycotoxin in Brazilian Corn by NIR Spectroscopy-Year 2019
1. Rouf TR, Prasad K, Kumar P (2016) Maize-A potential source of human nutrition and health: A review. Cogent Food And Agriculture 2(1).
2. Cowieson AJ (2005) Factors that affect the nutritional value of maize for broilers. Animal Feed Science and Technology 119(3-4): 293-305.
3. Ranum P, Pena Rosas J, Garcia Casal MN (2014) Global maize production, utilization, and consumption. Ann N Y Acad Sci 1312: 105-112.
4. CONAB (2020) Acompanhamento da safra Brasileira Grãos. p. 114.
5. Abimilho (2020) Associação Brasileira das Indústrias de Milho
[Internet].
6. Paterson R, Nelson Lima (2010) How climate change affects mycotoxins in food? Food Research International 43(7): 1902-1914.
7. Milani J (2013) Ecological conditions affecting mycotoxin production in cereals: A review. Vet Med (Praha) 58: 405-411.
8. Taniwaki MH, Pitt JI, Copetti MV, Teixeira AA, Iamanaka BT (2019)
Understanding Mycotoxin Contamination Across the Food Chain in
Brazil: Challenges and Opportunities. Toxins (Basel) 11(7): 411.
9. Sun XD, Su P, Shan H (2017) Mycotoxin Contamination of Maize in China.
Comprehensive Reviews in Food Science and Food Safety 16(5): 835-
849.
10. Ferrigo D, Raiola A, Causin R (2014) Plant stress and mycotoxin accumulation in maize. Agrochim Pisa LVII:116-127.
11. Munkvold GP, Arias S, Taschl I, Gruber Dorninger (2019) Chapter 9 - Mycotoxins in Corn: Occurrence, Impacts, and Management. AACC
International Press p. 235-287.
12. Alshannaq A, Yu JH (2017) Occurrence, Toxicity, and Analysis of Major
Mycotoxins in Food. Int J Environ Res Public Health 14(6): 632.
13. Giorni P, Bertuzzi T, Battilani P (2019) Impact of Fungi Co-occurrence on Mycotoxin Contamination in Maize During the Growing Season. Front
Microbiol 10: 1265.
14. Enyiukwu D, Awurum AN, Nwaneri JA (2014) Mycotoxins in stored agricultural products: implications to food safety and health and prospects of plant-derived pesticides as novel approach to their management. Greener J Microbiol Antimicrob 2: 32-48.
15. Omotayo OP, Omotayo AO, Mwanza M, Babalola OO (2019) Prevalence of Mycotoxins and Their Consequences on Human Health. Toxicol Res
35(1): 1-7.
16. Pereira KC, Santos CF (2011) Micotoxinas e seu potencial carcinogênico.
Ensaios e Ciência Ciências Biológicas, Agrárias e Saúde 15(4): 19.
17. Reyes W, Anguiano Sevilla C, Anguiano Estrella R, Jiménez Ortega L,
Torres Morán P, et al. (2018) Niveles de fumonisinas en rastrojo de maíz para consumo equino en el estado de Jalisco. Rev Mex Ciencias Pecu 9:
846-854.
18. Scussel VM, Savi GD, Costas LLF, Xavier JJM, Manfio D, et al. (2014)
Fumonisins in corn (Zea mays L.) from Southern Brazil. Food Addit
Contam Part B Surveill 7(2): 151-155.
19. Marasas WF, Kellerman TS, Gelderblom WC, Coetzer JA, Thiel PG, et al. (1988) Leukoencephalomalacia in a horse induced by fumonisin B1 isolated from Fusarium moniliforme. Onderstepoort J Vet Res 55(4):
197-203.
20. Ross PF, Nelson PE, Richard JL, Osweiler GD, Rice LG, et al. (1990)
Production of fumonisins by Fusarium moniliforme and Fusarium proliferatum isolates associated with equine leukoencephalomalacia and a pulmonary edema syndrome in swine. Appl Environ Microbiol
56(10): 3225-3226.
21. Myburg RB, Dutton MF, Chuturgoon AA (2002) Cytotoxicity of fumonisin B1, diethylnitrosamine, and catechol on the SNO esophageal cancer cell line. Environ Health Perspect 110(8): 813-815.
22. Alizadeh AM, Rohandel G, Roudbarmohammadi S, Roudbary M,
Sohanaki H, et al. (2012) Fumonisin B1 contamination of cereals and risk of esophageal cancer in a high risk area in north eastern Iran. Asian
Pac J Cancer Prev 13(6): 2625-2628.
23. Rodrà guez Amaya DB, Sabino M (2002) Mycotoxin research in Brazil: the last decade in review. Brazilian J Microbiol 33: 1-11.
24. Kumar P, Mahato DK, Kamle M, Mohanta TK, Kang SG (2017) Aflatoxins:
A Global Concern for Food Safety, Human Health and Their Management.
Front Microbiol 7: 2170.
25. Mohsenzadeh M, Hedayati N, Riahi Zanjani B, Karimi G (2016)
Immunosuppression following dietary aflatoxin B1 exposure: a review of the existing evidence. Toxin Rev 35(3-4): 1-7.
26. IARC (1993) International agency for research on cancer, monographs on the evaluation of carcinogenic risks to humans, some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins, p. 489-521.
27. Diekman M, Green M (1992) Mycotoxins and reproduction in domestic livestock. J Anim Sci 70(5): 1615-1627.
28. Chang K, Kurtz HJ, Mirocha CJ (1979) Effects of the mycotoxin zearalenone on swine reproduction. Am J Vet Res 40(9): 1260-1267.
29. Benzoni E, Minervini F, Giannoccaro A, Fornelli F, Vigo D (2008) Influence of in vitro exposure to mycotoxin zearalenone and its derivatives on swine sperm quality. Reprod Toxicol 25(4): 461-467.
30. Bonnet MS, Roux J, Mounien L, Dallaporta M, Troadec JD (2012)
Advances in deoxynivalenol toxicity mechanisms: the brain as a target.
Toxins (Basel) 4(11): 1120-1138.
31. Sobrova P, Adam V, Vasatkova A, Beklova M, Zeman L, et al. (2010)
Deoxynivalenol and its toxicity. Interdiscip Toxicol 3(3): 94-99.
32. Rahmani A, Jinap S, Soleimany F (2009) Qualitative and Quantitative
Analysis of Mycotoxins. Comprehensive Reviews in Food Science and
Food Safety 8(3): 202-251.
33. Shanakhat H, Sorrentino A, Raiola A, Romano A, Cavella S, et al. (2018)
Current methods for mycotoxins analysis and innovative strategies for their reduction in cereals: an overview. J Sci Food Agric 98(11): 4003-
4013.
34. Jia B, Wang W, Ni XZ, Chu X, Yoon SC, et al. (2020) Detection of mycotoxins and toxigenic fungi in cereal grains using vibrational spectroscopic techniques: a review. World Mycotoxin Journal 13(2): 163-178.
35. Berardo N, Pisacane V, Battilani P, Scandolara A, Pietri A, et al. (2005)
Rapid Detection of Kernel Rots and Mycotoxins in Maize by NearInfrared Reflectance Spectroscopy. Journal of Agricultural and Food
Chemistry 53(21): 8128-8134.
36. (2020) Plataforma Olimpo.
37. Fandohan P, Hell K, Marasas WFO, Wingfield M (2003) Infection of maize by Fusarium species contamination with fumonisin in Africa. African
Journal of Biotechnology 2(12): 571-579.
38. Doohan FM, Brennan J, Cooke BM (2003) Influence of Climatic Factors on Fusarium Species Pathogenic to Cereals. European Journal of Plant
Pathology 109(7): 755-768.
39. Hirooka EY, Yamaguchi MM, Aoyama S, Sugiura Y, Ueno Y (1996) The natural occurrence of fumonisins in Brazilian corn kernels. Food Addit
Contam 13(2): 173-183.
40. Mallmann C, Santurio J, Almeida C, Dilkin P (2000) Fumonisin B1 in cereals and feeds from southern Brazil. Arq Inst Biol 68(1): 41-45.
41. Oliveira MS, Rocha A, Sulyok M, Krska R, Mallmann CA (2017) Natural mycotoxin contamination of maize (Zea mays L.) in the South region of
Brazil. Food Control 73: 127-132.
42. Orsi R, Corrêa B, Possi C, Schammass EA, Nogueira J, et al. (2000)
Mycoflora and occurrence of fumonisins in freshly harvested and stored hybrid maize. Journal of Stored Products Research 36(1): 75-87.
43. Sabino M, Prado G, Inomata E, Pedroso M, Garcia R (1989) Natural occurrence of aflatoxins and zearalenone in maize in Brazil. Part II. Food
Addit Contam 6(3): 327-331.
44. Sekiyama BL, Ribeiro AB, Machinski PA, Machinski Junior M (2005)
Aflatoxins, ochratoxin A and zearalenone in maize-based food products.
Brazilian J Microbiol 36: 289-294.
45. Rocha LO, Nakai VK, Braghini R, Reis TA, Kobashigawa E, et al. (2009)
Mycoflora and co-occurrence of fumonisins and aflatoxins in freshly harvested corn in different regions of Brazil. Int J Mol Sci 10(11): 5090-
5103.
46. de Lourdes Mendes de Souza M, Sulyok M, Freitas-Silva O, Costa SS,
Brabet C, et al. (2013) Cooccurrence of mycotoxins in maize and poultry feeds from Brazil by liquid chromatography/tandem mass spectrometry.
Scientific World Journal 2013: 427369.
47. Kebede H, Abbas HK, Fisher DK, Bellaloui N (2012) Relationship between aflatoxin contamination and physiological responses of corn plants under drought and heat stress. Toxins (Basel) 4(11): 1385-1403.
48. Milanez T, Valente Soares L, Baptista G (2006) Occurrence of trichothecene mycotoxins in Brazilian corn-based food products. Food
Control 17(4): 293-298.
49. Mallmann CA, Dilkin P, Mallmann AO (2015) Micotoxinas e micotoxicoses em suínos: situação atual no Brasil. In: Avanços em sanidade, produção e reprodução de suínos. (1st Ed.) Porto Alegre: Federal University of Rio
Grande do Sul, Pork Sector, Brazil, pp.221-237.
50. Mallmann C, Dilkin P, Mallmann A, Oliveira M, Coloma Z, et al. (2017)
Prevalence and levels of deoxynivalenol and zearalenone in commercial barley and wheat grain produced in Southern Brazil: an eight-year (2008 to 2015) summary. Tropical Plant Pathology 42: 146-152.
51. Geraldo MRF, Tessmann DJ, Kemmelmeier C (2006) Production of mycotoxins by Fusarium graminearum isolated from small cereals (wheat, triticale and barley) affected with scab disease in Southern
Brazil. Brazilian J Microbiol 37: 58-63.
52. Salay E, Mercadante A (2002) Mycotoxins in Brazilian corn for animal feed: Occurrence and incentives for the private sector to control the level of contamination. Food Control 13(2): 87-92.
53. Teixeira LC, Montiani Ferreira F, Locatelli Dittrich R, Santin E, Alberton GC (2011) Effects of zearalenone in prepubertal gilts. Pesquisa Veterinária
Brasileira 31(8): 656-662.
54. Kuiper Goodman T, Scott PM, Watanabe H (1987) Risk assessment of the mycotoxin zearalenone. Regul Toxicol Pharmacol 7(3): 253-306.












