Performance of Turkey Poults Fed Different Doses of Aflatoxins in the Diet
Published: January 7, 2008
By: R. H. Rauber, P. Dilkin, L. Z. Giacomini, C. A. Araujo de Almeida, and C. A. Mallmann
Key words: aflatoxin, turkey, performance, biochemical analysis
2007 Poultry Science 86:1620–1624
INTRODUCTION
Aflatoxins are secondary metabolites of some species of fungi of Aspergillus genus, such Aspergillus flavus and Aspergillus parasiticus. Many compounds are known as aflatoxins, but only aflatoxins B1, B2, G1, and G2 have toxigenic importance (OMS, 1983). Fungal development and aflatoxin production in foods depend on several factors, mainly conditions related with moisture, temperature, O2, and substrate composition (da Silva et al., 2000).
In the past 20 yr (1986 to 2006), the average aflatoxin incidence in Brazilian maize was about 50%, and the average concentration on the contaminated samples was 27 ppb in more than 35,300 samples analyzed in the southern region of Brazil (LAMIC, 2006). This concentration is 35% over the maximum limit (20 ppb) established in Mercosul for animal feed (Anvisa, 2002).
Aflatoxin sensibility varies among species. Turkey and geese are the most sensitive birds to aflatoxins (Arafa et al., 1981). Turkeys poisoned with aflatoxins generally develop inappetance, reduced spontaneous activity, unsteady gait, recumbence, anemia, and death. At necropsy, body condition is generally good, but there is generalized congestion and edema. Liver and kidney are congested, enlarged, and firm (Hoerr, 2003).
Aflatoxins present in contaminated feed are rapidly absorbed in the small intestine, affecting mainly the liver, leading to metabolic disorders. Fat degeneration and proliferation of biliary ducts induce bloody changes generally seen as the increase in hepatic enzyme activity, coagulopathies, and reduction in protein production (Fernandez et al., 1995). Many production parameters can be affected by aflatoxin poisoning, such as BW gain, feed consumption, plasmatic proteins, cholesterol, and mortality rate, even in turkey poults as in other avian species (Lanza et al., 1980; Giambrone et al., 1985a,b; Quist et al., 2000).
This work was carried out to evaluate the sensitivity of young turkey poults to different aflatoxin concentrations added to the diet from 1 to 42 d of age.
MATERIALS AND METHODS
Mycotoxins
The production of aflatoxins (B1, B2, G1, and G2) was done by culture of a A. parasiticus toxigenic strain (NRRL 2999) on rice, following the methodology developed by Shotwell et al. (1966). The culture material obtained was submitted to chromatographic analysis (Mallmann et al., 2000) to determine its aflatoxin concentration.
Feed Preparation
The feed for initial-phase turkey poults was formulated based on the nutritional requirements recommended by the NRC (1994), using basically corn, soybean meal, and vitamin-mineral premix with CP adjusted to 27%. The culture material with 743 mg/kg of total aflatoxins (B1 = 75.2%; B2 = 1.4%; G1 = 22.6%; G2 = 0.8%) was added to each ration to reach the desired aflatoxin concentration in the diet. Ration and water were provided ad libitum. The protocol for treatment of turkeys was as follows: 1) control feed without addition of aflatoxins, 2) 20 ppb of total aflatoxins, 3) 50 ppb of total aflatoxins, 4) 100 ppb of total aflatoxins, 5) 200 ppb of total aflatoxins, 6) 500 ppb of total aflatoxins, and 7) 1,000 ppb of total aflatoxins. Before and during the experiment, feedstuffs and diets were screened for aflatoxins, fumonisins, zearalenone, and trichothecenes. Assayed levels of these mycotoxins were below the detection limits of the technique used.
Birds and Experimental Protocol
Three hundred thirty-six 1-d-old BUTA 9 male turkey poults (Meleagris gallopavo) were randomly divided into 7 treatments with 6 replicate groups per treatment and 8 birds per replicate group. Turkeys were kept in 42 electrically heated battery brooders with raised wire floors from 1 to 42 d of age. Experimental diets were given to the turkeys from when they were 1 d old until the end of the experiment.
Body Performance
Birds were monitored daily to evaluate the behavior and intoxication clinical signs. Body weight and feed consumption were measured on d 21 and 42 of the experiment.
On the first day, the average BW was 58.39 g, and there was no difference among the treatments.
Necropsies and Pathologic Studies
Birds were killed after electric sensitization in 2 steps: half the birds were killed on d 21 of the experiment, and the remaining birds were killed on d 42 of the experiment. At necropsy, general aspects of the body and organs (form, color, and volume) were observed. The relative weights of the organs (liver, gizzard, heart, and bursa of Fabricius) and meat (breast and thighs) were measured. Segments of liver, kidney, and bursa of Fabricius were collected in formol (10%) for histopathological analysis. The organ fragments were processed following the usual histopathological techniques (Luna, 1968). Those parameters were evaluated on both d 21 and 42 of the experiment.
Clinical Biochemistry Analysis
Blood samples were collected from the jugular vein after birds were killed (21 and 42 d). Samples were centrifuged 30 min after collection, and serum was maintained under −4°C until biochemical testing was performed. Total plasmatic proteins, albumin, uric acid, cholesterol, alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase measurements were performed using commercial kits (Labtest Diagnostica SA, Vista Alegre, Lagoa Santa, Minas Gerais, Brazil).
Statistical Analysis
All data obtained in this work (feed consumption, BW, relative weight of organs, relative weight of meat, and clinical biochemistry measurements) were submitted to a simple regression analysis. Parameters that presented a significance level higher than or equal to 90% (P ≤ 0.10) were submitted to ANOVA (1-way) and Bonferroni’s multiple range test (P ≤ 0.05). Statistical analyses were done in Statgraphics 5.0 computer statistical program (Statgraphics Plus 5.0, Manugistics, Inc., Rockville, MD).
RESULTS
During the experiment, birds from groups receiving 100 to 1,000 ppb of aflatoxins showed clinical signs of aflatoxicosis, such as poor feathering, apathy, and pallor of feet and beak. Besides that, during the last week (35 to 42 d), some birds from groups receiving 500 and 1,000 ppb showed some convulsive crisis.
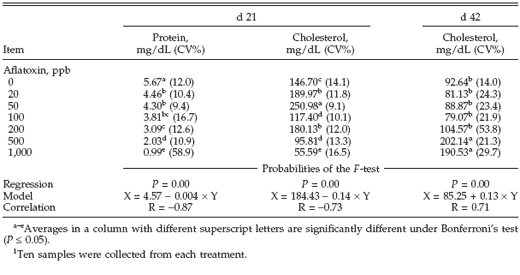
The feed consumption and BW of birds (21 and 42 d) is shown in Table 1. At d 21 of the experiment, the presence of aflatoxins until 50 ppb did not significantly affect feed intake. The lowest feed intake at 21 d was from birds that received the higher dose of aflatoxins (1,000 ppb). At this age, birds also showed a significant reduced BW when receiving 500 or 1,000 ppb total aflatoxins.
At 42 d of age, birds receiving 0, 20, 50, and 100 ppb of aflatoxins did not show a significant difference in feed intake and BW. However, turkeys from treatments that received 200 ppb of aflatoxins or more (200, 500, and 1,000) had a significant lower weight gain compared with the other treatments. At the 2 evaluated periods, both feed intake and BW had dose-dependent behavior, as seen in Table 1 (probabilities of the F-test).
Mortality in this experiment was about 10.1%. However, treatments that showed higher mortality were those that received 200 (18.7%), 500 (8.3%), and 1,000 (37.5%) ppb of aflatoxins. Mortality index had a strong correlation with aflatoxin doses (R = 0.88 and P < 0.01; data not shown).
In the first evaluation of the relative weight of organs (d 21 of the experiment), only the gizzard showed a significant correlation coefficient (P = 0.00) among the treatments (Table 2). This organ had a relative weight significantly higher in birds that received 500 and 1,000 ppb of aflatoxins. At the second evaluation (d 42), gizzard and liver showed significant increase in relative weight among treatments (P = 0.00). The behavior of gizzard relative weight in this evaluation was the same as in the former. However, liver relative weight was significantly increased only in birds that received 1,000 ppb of aflatoxins.
As seen in Table 3, total protein and cholesterol were significantly affected by aflatoxins in the diet at the fist evaluation (21 d). Both parameters were reduced in birds that received 1,000 and 500 ppb of aflatoxins. The second evaluation revealed that serum cholesterol had been significantly increased by aflatoxins (500 and 1,000 ppb). The effect of aflatoxins on other evaluated parameters was not significant at the simple regression test.
On d 21, the major gross lesions were observed in the livers of birds under the highest levels of aflatoxins (500 and 1,000 ppb). Those livers were smaller and yellowish. At 42 d, livers were also diminished; however, they were not yellowish. Histopathological examination (21 and 42 d) indicated proliferation of biliary ducts (moderated to severe) on livers from birds that received 200 ppb or more. Lesions on bursa of Fabricius were more discrete and appeared on birds that received 500 and 1,000 ppb as a decrease in the number of follicular cells. No significant lesions on kidney were found.
Table 1. Feed intake and BW of turkey poults poisoned with different doses of aflatoxins during d 21 and 42 of the experiment
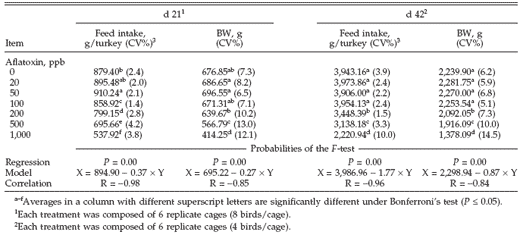
Table 2. Relative weight of liver and gizzard of turkey poults poisoned with different doses of aflatoxins during d 21 and 42 of the experiment
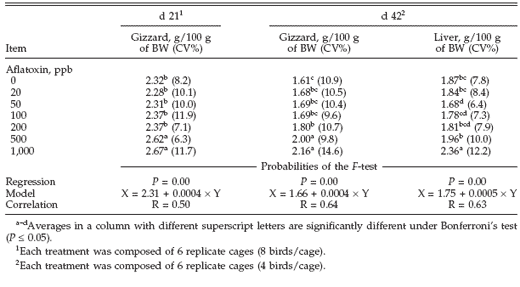
Table 3. Total protein and cholesterol levels in serum of turkey poults poisoned with different doses of aflatoxins during d 21 and 42 of the experiment1
DISCUSSION
Aflatoxins produce a reduction inBW gain (Giambrone et al., 1985a), severe economic losses, and health problems in the poultry industry because of their toxicity and frequency of occurrence in feedstuffs (Miazzo et al., 2005; LAMIC, 2006). The current study demonstrated the toxicity of several doses of aflatoxins added to the feed of turkey poults from 1 to 42 d of age. Several factors contribute to the lower weight gain in turkey poults, either at 21 and 42 d, such as reduced feed intake, reduction in liver protein synthesis, and decrease in lipoid metabolism.
In addition, Osborne and Hamilton (1981) investigated the effects of dietary aflatoxins on various digestive enzymes in broiler chickens and found the specific activities of pancreatic amylase, trypsin, and lipase to be approximately one-half of the activities observed in control chicks. This reduction may be due to the decreased protein synthesis, generally observed in chronic aflatoxicosis.
Joffe (1970) concluded that 6,650 ppb is the minimum aflatoxin dose that can significantly reduce the weight gain in turkey poults (during the first 21 d). Besides this fact, other authors have found similar results to the current research. Hamilton et al. (1972) found that 250 ppb is the minimum concentration that can significantly affect BW gain in turkeys. According to Witlock and Wyatt (1981) and Arafa et al. (1981), the minimum doses are 500 and 700 ppb, respectively. Giambrone et al. (1985a) poisoned turkey poults during 35 d with a maximum dose of 800 ppb. At the end of the experiment, they concluded that 400 ppb is the minimum dose to cause a significant loss in BW. In another study, Giambrone et al. (1985b) used turkeys during 35 d of age, and birds that received both 500 and 1,000 ppb were all dead. Data shown by Witlock and Wyatt (1981) are similar to our results.
The reduction in BW gain in turkeys that received 1,000 ppb of aflatoxins was about 38% compared with the control group at d 21 and 42 of the experiment. Broiler chickens poisoned with 3,000 ppb of aflatoxins showed a reduction of 37 and 27% when compared with the control group at 21 and 42 d, respectively (Giacomini et al., 2006). These results show that turkey poults are 3 to 6 times more sensitive to aflatoxins than broilers.
The main aflatoxin action mechanism is the reduction on the function of liver, primarily inhibition of the synthesis of proteins. The lipidic metabolism is also affected due to the reduction on enzymes synthesis and activity, mainly in chronic exposures (Hussein and Brasel, 2001). Both hypo- and hypercholesterolemia have been associated with liver diseases or liver damage (Campbell and Coles, 1986). Quist et al. (2000) showed that total protein levels are significantly reduced in turkeys poisoned with 200 and 400 ppb of aflatoxins, whereas cholesterol levels were diminished only in those birds that received 200 ppb of aflatoxins. In addition, other researchers (Witlock and Wyatt, 1981) have found a decrease in total protein levels with aflatoxin contamination equal or higher than 125 ppb.
The singular yellowish color of the liver is commonly observed in cases of chronic aflatoxicosis in the field and has been previously described in other studies with aflatoxins in broilers (Miazzo et al., 2005).
In the present study, our findings show that the presence of aflatoxins in doses equal or higher than 200 ppb affect turkey performance during the evaluated period (1 to 42 d). The effect of those toxins on BW, feed consumption, relative weight of gizzard and liver, mortality, and total protein and cholesterol levels on serum is dependent on the dose of aflatoxins at the diet. Turkey poults are very sensitive to aflatoxin poisoning, and important economic losses can occur mainly in industrial production, when micotoxicological management is not adequately conducted.
REFERENCES
Anvisa. 2002. Regulamento tecnico Mercosul sobre limites maximos de aflatoxinas admissıveis no leite, amendoim e milho. GMC/Res. No. 25/02. Mercosul, Buenos Aires, Argentina.
Arafa, A. S., R. J. Bloomer, H. R. Wilson, C. F. Simpson, and R. H. Harms. 1981. Susceptibility of various poultry species to dietary aflatoxin. Br. Poult. Sci. 22:431–436.
Campbell, T. W., and E. H. Coles. 1986. Avian clinical pathology. Pages 279–301 in Veterinary Clinical Pathology. 4th ed. E. H. Coles, ed. Saunders, Philadelphia, PA.
da Silva, J. B., C. R. Pozzi, M. A. B. Mallozzi, E. M. Ortega, and B. Correa. 2000. Mycoflora and occurrence of aflatoxin B1 and fumonisin B1 during storage of Brazilian sorghum. J. Agric. Food Chem. 48:4352–4356.
Fernandez, A., M. T. Verde, J. Gomez, M. Gascon, and J. J. Ramos. 1995. Changes in the prothrombin time, haematology and serum proteins during experimental aflatoxicosis in hens and broiler chickens. Res. Vet. Sci. 58:119–122.
Giacomini, L., F. A. Fick, P. Dilkin, C. A. Mallmann, R. H. Rauber, and C. Almeida. 2006. Desempenho e plumagem de frangos de corte intoxicados por aflatoxinas. Cieˆnc. Rural 36:234–239.
Giambrone, J. J., U. L. Diener, N. D. Davis, V. S. Panangala, and F. J. Hoerr. 1985a. Effects of aflatoxin on young turkeys and broiler chickens. Poult. Sci. 64:1678–1684.
Giambrone, J. J., U. L. Diener, N. D. Davis, V. S. Panangala, and F. J. Hoerr. 1985b. Effect of purified aflatoxin on turkeys. Poult. Sci. 64:859–865.
Hamilton, P. B., H. T. Tung, J. R. Harris, J. H. Gainer, and W. E. Donaldson. 1972. The effect of dietary fat on aflatoxicosis in turkeys. Poult. Sci. 51:165–170.
Hoerr, F. J. 2003. Mycotoxicoses. Pages 1103–1132 in Diseases of Poultry. 3rd ed. Y. M. Saif, H. J. Barnes, A. M. Fadly, J. R. Glisson, L. R. McDougald, and D. E. Swayne. Iowa State
Press, Ames.
Hussein, H. S., and J. M. Brasel. 2001. Toxicity, metabolism and impact of mycotoxins on humans and animals. Toxicology 167:101–134.
Joffe, A. Z. 1970. Feeding tests with ducklings, turkey chicks and rabbits and the effects of aflatoxin on these animals. Mycopathol. Mycol. Appl. 40:49–61.
LAMIC. 2006. Subject: Results. http://www.lamic.ufsm.br/ resultados.html Accessed Jul. 2006.
Lanza, G. M., K. W. Washburn, and R. D. Wyatt. 1980. Variation with age in response of broilers to aflatoxin. Poult. Sci. 59:282–288.
Luna, G. C. 1968. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. 3rd ed. McGraw-Hill, New York, NY.
Mallmann, C. A., J. M. Santurio, C. A. Almeida, F. Fontana, C. P. Mostardeiro, and E. C. Stefanon. 2000. Automation of the analytical procedure for the simultaneous determination of aflatoxins AFB1, AFB2, AFG1 and AFG2. Page 35 in Proc. X Int. IUPAC Symp. Mycotoxins and Phycotoxins, Sao Paulo, Brazil. Inst. Adolfo Lutz, Sa˜o Paulo, Brazil.
Miazzo, R., M. F. Peralta, C. Magnoli, M. Salvano, S. Ferrero, S. M. Chiacchiera, E. C. Q. Carvalho, C. A. R. Rosa, and A. Dalcerol. 2005. Efficacy of sodium bentonite as a detoxifier of broiler feed contaminated with aflatoxin and fumonisin. Poult. Sci. 84:1–8.
NRC. 1994. Nutrient Requirements of Poultry. 9th rev. ed. Natl. Acad. Press, Washington, DC.
OMS. 1983. Criterios de salud ambiental. Org. Panam. Salud, Cidade do México.
Osborne, D. J., and P. B. Hamilton. 1981. Decreased pancreatic digestive enzymes during aflatoxicosis. Poult. Sci. 60:1818– 1821.
Quist, C. F., D. I. Bounous, J. V. Kilburn, V. F. Nettles, and R. D. Wyatt. 2000. The effect of dietary aflatoxin on wild turkey poults. J. Wildl. Dis. 36:436–444.
Shotwell, O. L., C. W. Hesseltine, R. D. Stubblefield, and W. G. Sorenson. 1966. Production of aflatoxin on rice. Appl. Microbiol. 14:425–428.
Witlock, D. R., and R. D. Wyatt. 1981. Effect of dietary aflatoxin on hemostasis of young turkey poults. Poult. Sci. 60:528–531.
Authors: R. H. Rauber, P. Dilkin, L. Z. Giacomini, C. A. Araujo de Almeida, and C. A. Mallmann
Laboratory of Micotoxicological Analysis, Department of Preventive Veterinary Medicine, Federal University of Santa Maria, 97.105-900, Brazil
Related topics:
Recommend
Comment
Share

Would you like to discuss another topic? Create a new post to engage with experts in the community.