1. Introduction
Aflatoxin M1 (AFM1), it is one of five principal metabolites results from the hydroxylation process of Aflatoxin B1 (AFB1). Reaction of enzyme oxidase is associated to cytochrome P450 of the microsomes within the hepatocytes [1–6]. During this oxidative process, AFB1 is successively transformed into two intermediates, Aflatoxicol (AFL) and Aflatoxicol M1, before turning into AFM1, and in this form it is excreted through milk or eggs [3]. Some authors estimate that between 0.3% and 6.2% of AFB1 ingested by cattle it is transformed into AFM1 on the liver to be later excreted on milk [2,4,7,8].
AFM1 is considered as a possible carcinogenic agent for humans [1]. In regard to other effects on health, skin diseases and liver disorders were diagnosed in sub-Saharan children fed with breast milk contaminated by AFM1 and AFL in amounts equal to or greater than 0.100 μg/kg [9]. It was also found that the presence of both aflatoxins in serum of children with Kwashiorkor was higher than in children without this nutritional disorder [9]. Besides, the intake of AFM1 contaminated milk can have immunosuppressive effects on infants, as well as cause delay in height-for-age and weight-for-age deficiency [8,10,11]. Children under 15 years old are especially vulnerable to mycotoxin exposure, including AFM1, mainly because they have low capacity to eliminate toxins, a rapid growth rate, a high intake of food and water per unit of body weight [11].
Another important aspect is that AFM1 contamination of cow´s milk is spread geographically. From the set of available data by country, the parameters quantified in Costa Rica [12], Ethiopia [8], Jordan [13,14] and Iran [2] stand out either by the high prevalence of contamination or because of the relatively high AFM1 content in raw milk samples.
There is evidence of seasonal variation both in the proportion of cases and in the detected levels of AFM1 in milk. Usually, the values increase during the winter or in the dry season of the tropics, precisely when cattle are fed with possibly contaminated feedstuffs and silages. On the contrary, a decrease in occurrence and levels of AFM1 is observed just during spring and summer or in the rainy season of tropical or subtropical zones, when enough pasture is available to feed livestock [2,5,6,15,16].
Previously, it has also been indicated that the high prevalence of contamination and relatively high levels of AFM1 are characteristic of countries with dry climatic conditions or seasons with long periods of drought, since these conditions favor the growth of molds and, therefore, contamination of food for livestock by AFB1 [3,15].
In El Salvador, there is no information on the levels or occurrence of contamination by AFM1 in samples of cow´s milk. Neither is known about the territorial distribution or variations of these parameters associated with drought, in a country located in the Central American Dry Corridor and prone to this climate phenomenon [17]. On the other hand, milk consumption in El Salvador has also increased from 135 million liters in 2008 to 167 million liters in 2014, at an average annual growth rate of 3.3% [18], driven by the "Vaso de Leche Escolar" governmental program, which benefits approximately one million students of more than 2900 public schools [19]. Probable unsafe milk intake by children should be consider as a serious health risk to be concerned about.
Therefore, this work quantified the levels of AFM1 in raw cow milk during the dry-rainy transitional period of two consecutive years and the variation in occurrence associated with the meteorological drought in El Salvador was determined.
2. Material and methods
2.1. Study type and sampling
This was a longitudinal descriptive-observational study, conducted in May-June 2016 and continued in May-June 2017, months corresponding to the transition from dry to rainy season, with a total sample for convenience of 107 non-specialized milk producing units of four municipalities of El Salvador: San Pablo Tacachico in the Central region (2016, n = 27; 2017, n = 24), Sonsonate in Southwest (2016, n = 24; 2017, n = 24), Chalatenango in the Northern region (2016, n = 17; 2017, n = 25), and Jocoro in the Eastern (2016, n = 39; 2017, n = 35).
Dairy cow feed management cycles between fresh pasture grasses during the rainy season and dry forage and feedstuff during the dry season. Thus, samples were collected during the transition between the dry to rainy seasons to maximize the time milk cows had been fed forage and feedstuff mixtures, rather than grazing on pasture.
The sampled production units are of double purpose - milk and meat (91%), an average of 17 cows in total, of which 11 were in milking, with average milk production of 74.8 kg per day and with an average yield of 6.2 kg/cow/day. The majority of the producers sells to intermediaries (86%) and the milk is destined mainly for the elaboration of cheeses and cream (85%), the rest is commercialized in a fluid way (15%).
The combination of feedstuffs and forages, in equal quantity or with predominance of the first item, are the forms of feeding of more recurrent use of milk producers (81%).
2.2. Ethical statement
This study did not involve taking tissue samples from humans, neither gathered clinical or personal data, therefore ethical approval or consent of participation, it´s not applicable on this work.
In relation to cows, these animals were not used as experimental subjects, nor tissue samples were extracted from them either, only milk as a secretion was collected. In consequence, Animal Research Guidelines were not required in this study
2.3. Preparation of the samples and extraction of AFM1
Of the 2250 ml collected per sample (2.3 kg), two aliquots of 15 ml each were taken, centrifuged at 3500 rpm, at 10 °C for 10 min, using a Hermle Z 366k refrigerated equipment. After cold separation, 1500 μl of the liquid was removed from under the grease layer, pouring it into Eppendorf Safe-Lock® colorless microcentrifuge tubes, following a specific procedure described by NEOGEN® Corporation (Lansing, Michigan, USA) [20]. An aliquot of 100 μl of this extracted liquid phase was directly tested to detect and quantify the AFM1, using an immunochemical technique called enzyme linked immunosorbent assay (ELISA).
2.4. Analysis of AFM1 in samples by competitive ELISA
NEOGEN® Corporation´s VERATOX® kit specific for AFM1 was used (100% cross-reactivity for AFM1, < 1% for AFB1, AFB2, AFG1 and AFG2), with a quantification range between 0.005 and 0.100 μg/kg. The kits were stored between 2 and 8 °C and, prior to use, were allowed to acclimate for one hour to reach room temperature (24 ± 2 °C).
The kits were used according to the manufacturer's specifications [20], and for the estimation of AFM1 concentration in the samples, expressed as μg/kg, the optical density values (absorbance) were calculated using NEOGEN VERATOX Software v.3.0.1.
Samples with values above the maximum control of the kit for AFM1, established at 0.100 μg/kg, were retested in duplicate, using a 1:2 or 1:4 dilutions to make an appropriate quantification of the contents, in concordance of the specifications of the reactive sets [21].
There is evidence to support a relationship between total Aflatoxins (AFs) in complementary cattle feeds and AFM1 in milk [1,2,4,8,23,24]. Taking into account this fact, two kg samples of each feedstuff and dry fodder for milking cattle were taken from those farms with milk AFM1 levels equal to or higher than 0.100 μg/kg to detect and quantify AFs contents that exceed 20 μg/kg maximum limit settled by the Codex Alimentarius Commission [22]. The AFM1 sampling criteria is based on children exposure to this aflatoxin in equal to or higher than 0.100 μg/ kg is associated with infancy diseases in tropical and subtropical regions [9].
In a previous study, the procedures for taking samples for the extraction of AFs with aqueous solutions of methanol (70%), as well as their quantification by the Competitive Direct ELISA method, were specifically described using NEOGEN® VERATOX® specific kits for the AFs [25]. Similar to AFM1, those samples that exceeded the maximum limit of quantification of the kit for AFs (50 μg/kg), were retested in duplicate through 1:2 or 1:4 dilutions, to properly quantify the contents, according to the specifications of the reactive sets [21].
2.5. Analytical method performance assessment
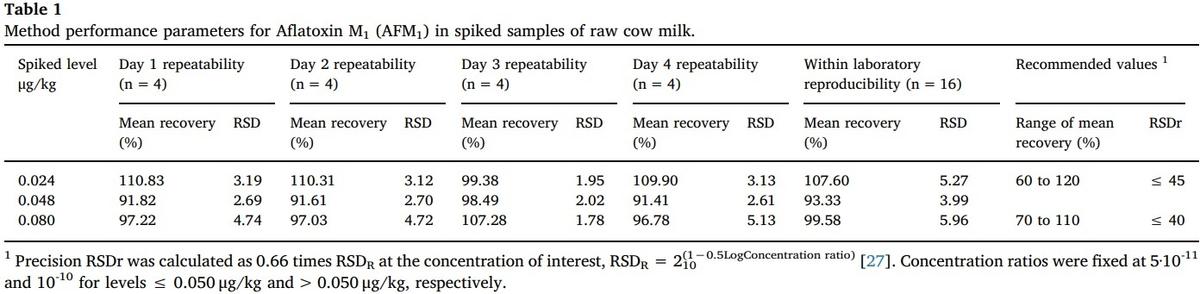
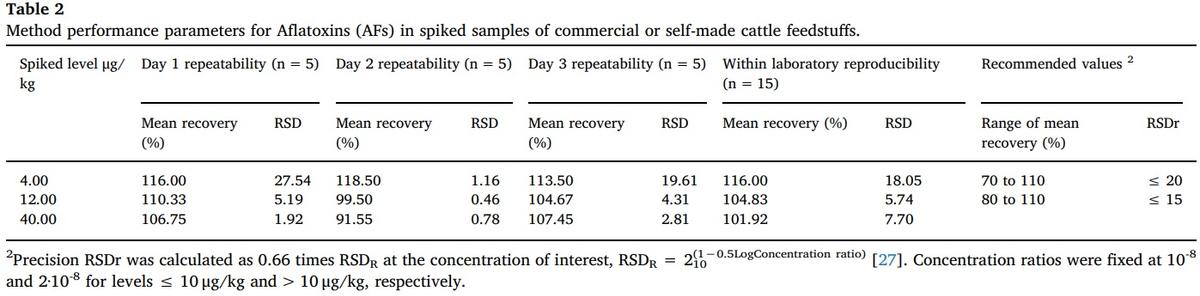
Evaluation of the method performance was carried out by spiking samples of both milk and feedstuff with AFM1 and AFs of known concentrations, respectively. The extraction of the toxins and their quantification was done in the same way as described for the samples, except that the milk samples were spiked with AFM1 solutions at concentrations of 0.024, 0.048 and 0.080 μg/kg, similar to other previous validations carried out [2]. Feedstuff samples were spiked with AFs solutions at concentrations of 4, 12 and 40 μg/kg.
The AFM1 assay was carried out four times for each level for four consecutive days (Table 1), while AFs assay was tested five times for each concentration for three consecutive days (Table 2). In both cases, the analyzes were performed with the same instruments, but using different reactive kits every day. The recovery was calculated dividing the measured content of a sample between the spiked level and multiplying by 100 [2], the mean recovery is the simple average of the set of recovery values obtained by day and by concentration of the spiking [2].
The accuracy of both repeatability and reproducibility was calculated by means of the Relative Standard Deviation (RSD) of the mean recovery [2,26]. The mean recovery values between the days tested did not show significant differences, both for the AFM1 (F = 0.210, 3 g l., p = 0.889) and for the AFs (F = 1.166, 2 g l., p = 0.321). In addition, repeatability such as reproducibility were found to be within the range of values recommended for AFM1 and AFs [26]. In the case of the values for AFM1, those were also similar to the average recovery and accuracy obtained in other works [8,16].
2.6. Maximum levels of Aflatoxins
In El Salvador, since there is no national regulation for AFM1 in milk as a raw material, the maximum level established by The European Commission at 0.050 μg/kg was adopted for this study [27]. This upper limit provides an adequate margin of safety to protect human health based on the ALARA principle "As low as reasonably achievable" [27]. This criterion applies to any compound that is a possible genotoxic human carcinogen, as in the case of AFM1, considering that exposure to any level of this Aflatoxin could represent a risk to the health of consumers [28]. In the case of AFs, the maximum level of 20 μg/kg was assumed, established by the Codex Alimentarius Commission [22].
2.7. Meteorological drought indicator
To determine the levels variation of the occurrence of AFM1 associated with the deficit of precipitation in El Salvador, the consecutive number of dry days during the rainy season for each locality sampled was used as indicator of intensity of meteorological drought [29], based on the cumulative rainfall record available at the national climate station network nearest the four locations sampled.
3. Statistical analysis
The statistical significance for differences between proportions was determined through the Chi Square test, and for mean values Student t test was used, establishing a significance of (p < 0.05) for both tests. In the case of the test for mean, the Levene test was previously applied for equality of the variances. In case the data did not comply with the assumption of homogeneity of the variances, the median test was used to determine statistical significance between differences, always at the level of p < 0.05. To establish the association or relationship between variables, the Pearson coefficient was calculated or the curvilinear regression analysis was made, respectively. The application of the tests and the generation of the figures was done with the IBM SPSS Statistics v.24 for Windows program.
4. Results
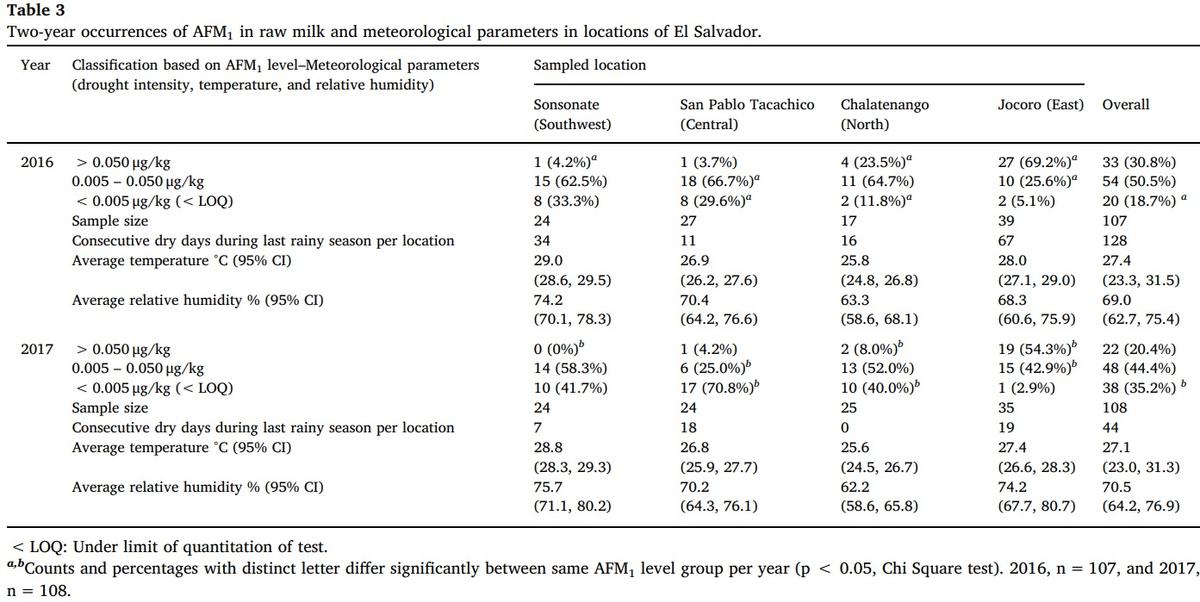
Occurrence of samples of raw milk positive to AFM1 for the years 2016 and 2017, in four different locations producing dairy products in El Salvador is presented in Table 3; the consecutive dry days during the rainy season as an indicator of meteorological drought intensity, average temperature and relative humidity are also shown for same localities.
The occurrence of AFM1 positive samples was significantly higher in a drought year (2016) compared with a non-drought year (2017) for all sampled locations (χ2 = 76.162, 11 g l, p < 0.001, Table 3). Concomitantly, the number of negative samples was significantly lower in a drought year (2016) in comparison to a year without (2017) for all locations (χ2 = 7.491, 2 g l, p < 0.05, Table 3) and quite particular on San Pablo Tacachico (χ2 = 37.031, 2 g l, p < 0.001) and Chalatenango (χ2 = 24.517, 2 g l, p < 0.001).
In general terms, when comparing the drought year (2016) with the non-drought year (2017), there was a difference of 16.5 percentage points in the occurrence of positive samples to AFM1 (81.3% vs. 64.8%); consequently, the proportion of samples that exceeded the level of 0.050 μg/kg of AFM1, also showed a difference (30.8% vs. 20.4%). Similarly, negative samples had a difference between 2016 and 2017 (18.7% vs. 35.2%, Table 3).
The general trend described above it is based on the average positive correlation that exists between the number of AFM1 positive samples and the number of consecutive dry days during the rainy season (Pearson r = 0.480, F = 63.628, p < 0.001, n = 215). It was also found that the number of samples positive to AFM1 are significantly correlated to the average annual temperature (Pearson r = 0.222, F = 11.034, p < 0.01, n = 215). These findings would indicate that as the intensity of the drought and the annual average temperature increase, the prevalence of positive cases to that Aflatoxin raises too.
Levels of AFM1 in milk samples collected in 107 dairy farms of four different locations during a drought (2016) and during another nondrought year (2017) are presented in Fig. 1. No significant differences were detected for the localities of Sonsonate, San Pablo Tacachico and Chalatenango between 2016 and 2017, maintaining their values below 0.040 μg/kg. In Jocoro on the contrary, there was a significant difference between average levels quantified during the drought year (0.100 μg/kg) and non-drought year (0.059 μg/kg) (Student t = 3.251, 56 g l, p < 0.01). It should be noted that Jocoro is an endemic area for the presence of AFM1 in raw milk, because it maintains an occurrence of positive samples that exceeds 30% (Table 3) and average levels above 0.059 μg/kg (Fig. 1), such condition could be associated with the fact that this locality is located in the eastern end of the Dry Corridor of El Salvador where the drought is more severe.
In general terms, average AFM1 level of the four localities measured during 2016 (0.056 ± 0.007 μg/kg) was significantly higher than mean value determined during 2017 (0.039 ± 0.004 μg/kg, Student t = 2.101, 143 g l, p < 0.05), as shown in Fig. 1.
Consistent with the overall locations trend shown in Fig. 1, the variations on AFM1 levels are significantly associated with the number of consecutive dry days in the rainy season, observed between 2016 and 2017 (Pearson r = 0.535, F = 62.082, p < 0.001, n = 157), which would indicate that as the intensity of the drought increased, the average levels of AFM1 did, as well. The levels of AFM1 are also associated with the average annual temperature recorded between 2016 and 2017, although in a positive-low but significant way (Pearson r = 0.162, F = 4.159, p < 0.05, n = 157).
Fig. 1. Two-year comparison of AFM1 levels in raw cow milk samples. Aflatoxin contents are showed per year, location, and overall. Numbers inside bars are means and whiskers indicate ± 1 SEM (n = 107 in 2016, n = 108 in 2017). Year bars with distinct letters differ significantly within each location group (p < 0.05, Student t test).
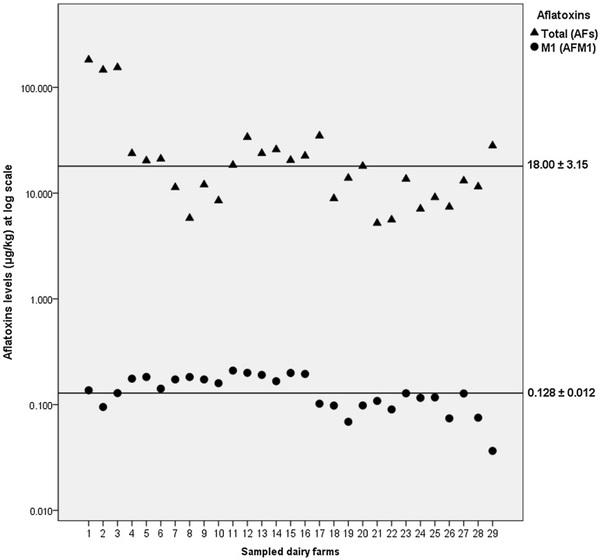
Fig. 2. Matching between quantified levels of AFM1 in raw milk and AFs in feedstuffs. Samples were taken from 29 dairy farms in El Salvador. Solid lines represent median levels ± standard error of AFs (upper position) and AFM1 (lower position) respectively.
Contents of AFs also showed a significant difference between median values detected in the drought year (22.51 ± 6.25 μg/kg, range = 5.8–182.4 μg/kg, n = 17) and a non-drought year (10.30 ± 2.00 μg/kg, range = 5.2–28.1 μg/kg, n = 12; χ² = 13.079, 1 g l, p < 0.001), similar as it was demonstrated in AFM1 levels.
Correspondence between the levels of AFM1 and AFs was also observed (Fig. 2). Average AFM1 content in milk represents 0.98 ± 0.13% (n = 29) of mean AFs level quantified in the feedstuffs. During 2016, the year most affected by drought, milk AFM1 levels equal to or higher than 0.100 μg/kg were significantly related to AFs contents in feedstuffs used by 17 dairy farms, demonstrated by means of a linear regression described by the equation AFM1= -3.78·10−4 AFs + 0.182 (r2 = 0.392, F = 9.662, p < 0.01, n = 17)
In 2017, the year least affected by the drought, milk AFM1 levels were also significantly related to AFs contents in feedstuffs of 12 dairy farms and represented by the exponential function AFM1 = 0.137·exp (-0.036AFs) (r2 = 0.414, F = 7.070, p < 0.05, n = 12).
All datasets generated and analyzed during the current study are available at the Mendeley Data site: http://data.mendeley.com/ datasets/d4ztwn632m/2 [30].
5. Discussion
There is a variation in the occurrence of cases of contamination by AFM1 associated with drought conditions, characterized by a greater number of consecutive days without rain and a higher average environmental temperature. In this regard, significance could be demonstrated in the greater number of positive samples in the four surveyed locations in a year with drought effects compared to another year without that phenomenon. Localities of Sonsonate, Chalatenango and Jocoro had an increased number of cases that exceed 0.050 μg/kg in 2016 compared to 2017.
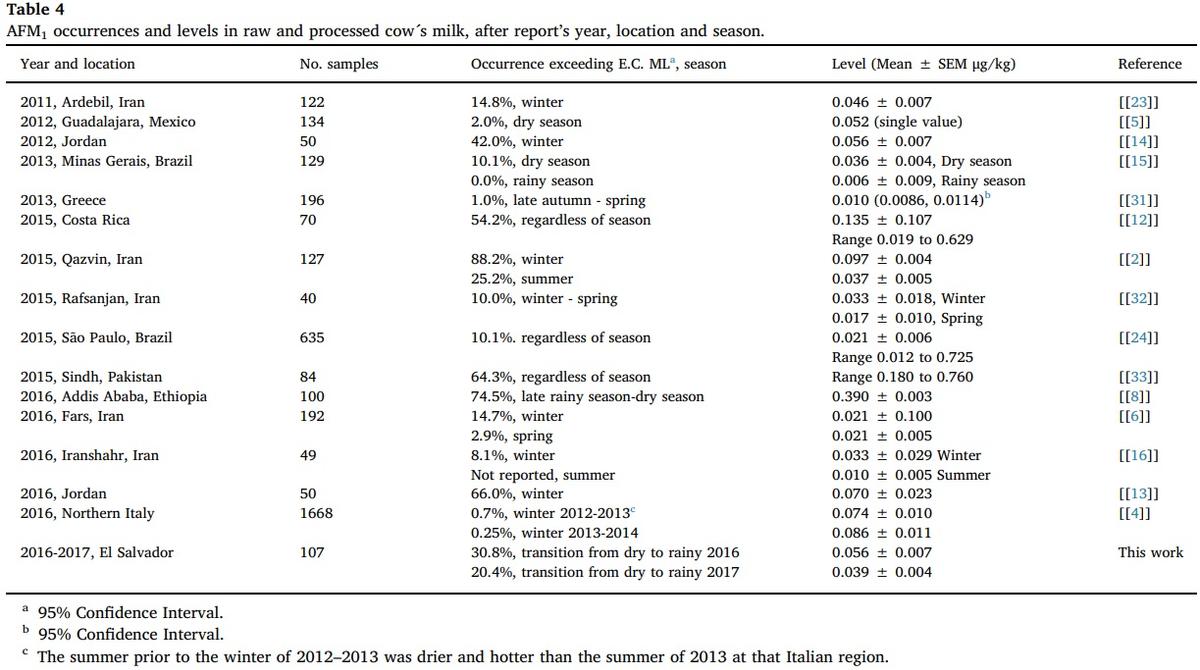
Regardless of the year, the overall occurrence of AFM1 cases reported in this work is higher than most of studies shown in Table 4, except for those ones from Middle East countries, Ethiopia and Costa Rica [2,8,12–14,33]. Nevertheless, when considering only Jocoro, located in the Salvadoran region sternly affected by drought and heat, then AFM1 occurrence values (54% and 69%) are only lower than prevalence cases quantified in Pakistan, Iran, and Ethiopia (Tables 3 and 4).
In general, the average proportion of cases with levels exceeding the limit of 0.050 μg/kg exceeded 30% in the year affected by drought, to a proportion of 20% in one without affectation. Concomitantly, the proportion of negative samples to AFM1 went from 18.7% to 35% between the year with drought to the other without that condition. This bimodal variation would indicate that, in drought conditions, the proportion of samples contaminated by AFM1 and that exceed the limit of 0.050 μg/kg tends to increase. Consistent with the above, it was shown that the number of cases positive to AFM1 are significantly associated both to the number of consecutive dry days during the rainy season (p < 0.001), and to the average annual temperature (p < 0.01).
At the base of this increase in cases would be prevailing climatic conditions in regions with extended periods of drought, which favors the growth of molds that contaminate food for livestock [3,15] and El Salvador for being located in the Dry Corridor of Central America, it is a country prone to suffer from these conditions of deficit precipitation [29].
Although the average contents of AFM1 in three of the four localities sampled are below the maximum limit established by The European Commission (> 0.050 μg/kg) [27], the values found in Jocoro always exceed that limit. At the base of this behavior is the fact that this place is located in the eastern part of the country, which has the greatest impact due to drought [17], besides the difference in the average annual temperature was +0.6 °C in 2016 compared to 2017. It also follows that the area does not produce enough ingredients for the preparation of feedstuff, which is why the raw material must be collected regardless of the place and source of origin, usually without making an adequate selection to deal with the shortage caused by drought.
Irrespective of the year, the overall mean level of AFM1 of this work is similar to or slightly higher than most of studies shown in Table 4, with the exception of those also reported from Middle East countries, Ethiopia, Costa Rica, and Northern Italy [2,4,8,12,13,33]. Again, when only Jocoro is considered, a Salvadoran location severely affected by drought and heat, then AFM1 average levels (0.059 ± 0.006 μg/kg and 0.100 ± 0.011 μg/kg) are only lower than mean values reported from Costa Rica, Pakistan, and Ethiopia (Fig. 1 and Table 4).
The average level of AFM1 of the four localities sampled varied in a similar way to the occurrence of contamination cases, being significantly higher during the year of drought with respect to the year without that affectation (p < 0.05). In addition, it was demonstrated that AFM1 levels are significantly associated with both the number of consecutive dry days during the rainy season (p < 0.001) and annual average temperature (p < 0.05). The significance in the association of the occurrence values as well as the AFM1 levels with the precipitation deficit indicator and temperature, would indicate that as the intensity of the drought and heat increase, both the number of positive cases and AFM1 contents also augment.
The above evidences are consistent with what has been indicated in other studies, in respect that the occurrence of contamination cases or relatively high levels of AFM1 are characteristic of regions with high or moderate temperatures and low rainfall (Table 4), which in repercussion promotes fungal outcrop in feedstuff [3,15].
Seasonal variations in the occurrence and in the average levels of AFM1 described in other similar studies are shown in Table 4. As it can be seen in this list, five reports describe an increasing trend in both AFM1 prevalence and mean contents during the northern winter or in the dry season of the tropics [2,6,15,16,32], when cattle are mostly fed with possibly contaminated feedstuffs and silages. Conversely, same studies also describe a decreasing trend that can be observed in both the occurrence and mean levels of AFM1 [2,6,15,16,32], when enough pasture is available to feed livestock, just during northern spring and summer or in the rainy season of tropical zones (Table 4).
Regarding AFs in feedstuff analyzed in this study, levels were detected in a range between 5.8 to 182.4 μg/kg during the year with drought, and another that fluctuated from 5.2 to 28.1 μg/kg, during the year with non-deficit precipitation. A recent evaluation in Pakistan detected AFB1 contents in cattle feeds that averaged 29.3 μg/kg and 21.9 μg/kg, respectively [34]. In another work carried out in Ethiopia, 114 samples of feedstuff were tested to detect AFB1, quantifying an average level of 91 μg/kg [8]. These levels are within range of the quantified AFs in the feedstuff samples taken in El Salvador
Such a comparison is possible because AFB1 is the most frequent type of the four that form the AFs conjunct [1,20,35], so that when quantifying AFs, the variant B1 is measured indirectly. In addition, the sampling in El Salvador was conducted between May and July, coinciding with the maximum values found in Pakistan [34] and Ethiopia [8], between the months of June and September.
The median levels of AFs, both in commercial and self-prepared feedstuff, varied in a similar way to those of AFM1 in milk, being significantly higher in the samples collected in the year with drought compared to those obtained in the year without that effect (p < 0.001). Both kinds of cattle feedstuff have common feed ingredients such as cornmeal, soymeal, peanut meal, palm kernel meal, wheat bran, molasses, calcium carbonate, and common salt. Previous studies on AFs or AFB1 contaminated feedstuffs and their ingredients have shown that corn is the most susceptible of them [27,34,36], probably due to inadequate postharvest practices such as drying, cleaning or sorting, and poor storage conditions [34,36,37]. In addition, temperature increase and rain decrease can promote suitable conditions for development of fungal contamination of cereal such as maize [4,27,34].
In places prone to drought, it is usual for grains and fodder used for the preparation of feedstuff to be gathered without making a previous selection, a practice carried out to deal with the shortage of raw material due to relatively prolonged and frequent periods of drought. In addition, the materials stored for this purpose tend to stay longer than necessary under inadequate ventilation conditions, with high relative humidity and high temperature, which promotes the outcrop of fungi and the consequent contamination by AFs.
Statistical significance was shown in the relationship between the levels of AFM1 in raw milk and the contents of AFs in feedstuff, both during the dry period (r2 = 0.392, p < 0.01), and non-deficit precipitation (r2 = 0.414, p < 0.05). In both cases, the correlation between the two Aflatoxins (r = 0.62), determined in this study, was twice as high as that found in a previous study, also carried out during the dry season [8]. It was estimated that the average content of AFM1 detected in milk is 0.98% of the level of AFs in the concentrates, close to 1% determined in a study conducted in Ethiopia [8].
Some aspects should be considered in order to understand the relationship among AFM1, drought and hot conditions. In a previous study, daily mean temperature during growing season of forage maize was correlated with milk samples that exceeded maximum AFM1 levels by The European Commission [4]. The occurrence of AFM1 in milk is a carryover from AFs or AFB1 contamination of dairy cow feedstuffs, and the maize is the main ingredient of dairy animal feeds and the most susceptible to fungal contamination [27,38,39]. Furthermore, high air temperature and drought conditions increase the airborne inoculum of toxicogenic fungus in maize grain [27]. The drought and excessive heat evoke plant stress mainly during the reproductive stages [40], increasing kernel breakage susceptibility and insect damage of ears [27], thus both climate conditions can ease grain infection by mycotoxinproducing fungi, promote its growth and AFs production.
6. Conclusions
The changes observed both in the occurrence of contamination and in the contents of AFM1 in raw cow milk might be linked with drought and hot periods. This event would provide conditions for the number of positive cases and AFM1 levels to increase as the duration of the precipitation deficit and average annual temperature intensifies.
The AFs levels within feedstuff have similar behavior associated with drought, tending to increase as the duration of the precipitation deficit increases. At the base of this association would be the propitiation of high temperature conditions and low rainfall typical of drought with the outcrop of fungi and the consequent contamination of raw materials and cattle feed by AFs.
High temperature and drought effects on increasing values of AFM1 occurrence and content in cow milk are exerted through promoting toxicogenic fungi growth and its AFs production in maize grain, the main ingredient of dairy cow feedstuffs and the most susceptible to fungal contamination. So that, heat and drought stress conditions can evoke raising effects on both Aflatoxins level and occurrence due to AFM1 in milk is a carryover from AFs contaminated feedstuffs ingested by dairy cows.
The understanding of the relationship between AFs contents in feeds with AFM1 levels offers a knowledge base to prevent contamination of milk, by improving ventilation within storage devices or facilities, thereby preventing conditions of excessive relative humidity and high temperatures; Also, through the selection and prior cleaning of grains and forages collected to manufacture feedstuff and to practice the rotation or renewal of the stock, to avoid that the materials stored for this purpose remain more than necessary.
This article was originally published in Toxicology Reports, Volume 5, 2018, Pages 671-678. https://doi.org/10.1016/j.toxrep.2018.06.004. This is an Open Access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/BY-NC-ND/4.0/). 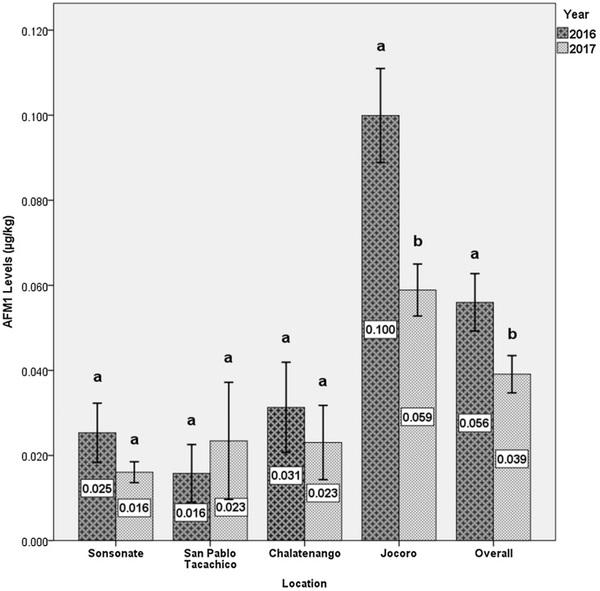






.jpg&w=3840&q=75)