Mycotoxins: Still present threat to Poultry Industries?
Published: June 19, 2019
By: Dr. Suresh F. Nipane / M.V.Sc.-Animal Nutrition, Suman Hatchery Ltd., Raipur (C.G.).
Introduction:
Mycotoxins are a diverse group of toxic secondary metabolites produced by certain moulds when they grow on agricultural products. They do not belong to a single class of chemical compounds, and they differ in their toxicological effects.
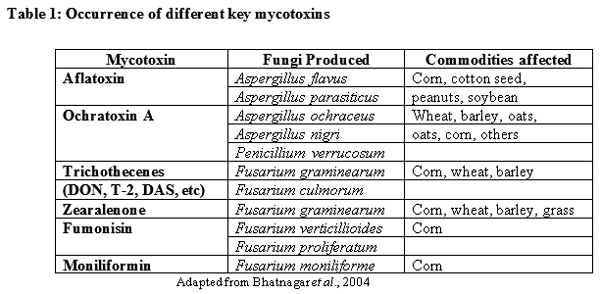
Although several hundred mycotoxins are known, the mycotoxins of most concern, based on their toxicity and occurrence, are aflatoxin, ochratoxin A, trichothecenes (DON, T-2 toxin, DAS, etc), zearalenone, fumonisin and moniliformin (Table 1)
What is a Mycotoxin?
- Secondary metabolites (chemicals) of a fungus that produce toxic results in another organism.
- Cytotoxic: Disrupt cell structures such as membranes, and processes such as protein, DNA, and RNA synthesis.
- Lack of visible appearance of fungus does not negate presence of mycotoxins. Toxins can remain in the organism after fungus has been removed.
- Less selective in organism selection, can cross plant species barrier.
- Can be heat stable, not destroyed by canning or other processes.
Brief History of Mycotoxins:
- Mycotoxin contamination has affected humans for thousands of years.
- In 7th and 8th century, festival for Roman God Robigus, protector of grain and trees was celebrated to stave off rust and mold.
- Middle Ages had outbreaks of ergotism.
- Only in last 30-40 years have scientists been able to isolate specific toxins from their fungal source.
- Research ideas and methodologies, in this field, change frequently, and data from 20 years ago are considered questionable
Mycotoxin Statistics:
- 300-400 mycotoxins presently identified, with more becoming evident as new isolation techniques are used.
- Most frequent toxins present are aflatoxin, DON, ZEN, fumonisin, and T-2 toxin, to name a few.
Factors affecting mycotoxin formation in the field:
As temperature and moisture levels are key factors for fungal growth and subsequent mycotoxin production, the climate plays a key role in the occurrence of mycotoxins. Three key agronomic factors have been shown to affect mycotoxin presence and concentration significantly:
- Crop presence and rotation
- Soil cultivation
- Crop and crop varieties
Morphology of Fungus:
- Range from single cells to fruiting bodies that form molds, mushrooms, smuts, and yeasts.
- Absorb nutrients from living or deceased organisms, contain no chlorophyll.
- If multicellular, they have tubular filaments called hyphae that branch out.
- Reproduce using spores
Modes of Spore Transmission:
- Airborne, wind or indoor ventilation systems.
- Attachment to insects of birds thus transmitted from plant to plant, or animal to animal, etc.
- Via transportation mechanisms such as trucks, crop machinery, etc.
Fungal Infection:
- Can occur at any stage in crop production.
- While in the field.
- During harvesting.
- While in silage and storage.
- Spores can lay dormant for months to years, waiting for positive conditions for germination.
Condition to Encourage:
Fungal Growth
- Relative humidity over 70%.
- Temperatures over 30 degrees Celsius for a period of a few days to a week.
- Stress to the affected plant, such as drought, flood, or insect infestation.
- High moisture content of crop (20% or higher).
- Must occur in conjunction, or fungal growth cycle will cease.
Locales of Mycotoxins:
- In North America, grain producing areas spread from central Canada to the southern parts of the US.
- These grains are then exported to Asia, the Pacific Islands, South and Central America, Europe, the Middle East, India and Africa.
Mycotoxin Health Hazards:
- Generally lower risk in well developed countries due to improved standards of living.
- High intake of affected product, usually in conjunction with limited amounts of other food sources.
- Greatest threat comes from long term exposure due to eating spoiled food or meat from animals fed contaminated feed.
Why Great Concern?
- Some mycotoxins are DEADLY at very small dosages.
- Some mycotoxins are carcinogenic.
- Some mycotoxins cause huge losses in productivity in animals.
Most fungi do not produce Mycotoxins:
- Many fungi are edible
- Mushrooms are fungi
- Moldy feeds may be degraded without presence of mycotoxin, or may be unaffected in value.
Feeds Most Susceptible to Fungi-producing Mycotoxins:
- Corn
- Wheat
- Oats
- Barley
- Sorghum
- Cottonseed
- Peanut meal
- Rye
Moldy grain is usually nontoxic:
- Competition between toxic and nontoxic molds.
- Entire mold population is not producing mycotoxin
- Conditions for growth are different for mold growth vs mycotoxin production
Molds that attack grain can:
- Decrease grade
- Kernel damage
- Odor
- Decrease milling quality
- Decrease seed germination
- Decrease dry matter
- Decrease feeding value (sometimes)
Mycotoxins can cause:
- Death
- Poor performance from low FI, ADG
- Respiratory problems
- Reproductive problems
- Liver, kidney or other organ damage
- Cancer
Factors causing variation in effects
- Species
- Breed
- Age
- Sex
- Nutritional status
- Other diseases
- Other mycotoxins
- Extent of exposure
Some mycotoxins are formed in the field, some in storage:
Storage conditions that favor production of mycotoxins:
- Temperature (40 – 900 F ; 4 – 320 C)
- Relative Humidity (> 70%)
- Moisture (22-23% in grain)
- Oxygen (1-2%)
Trichothecene Mycotoxins
- Nivalenol
- Deoxynivalenol
- T-2 toxin
- HT-2 toxin
- Diacetoxyscirpenol
- Triacetoxyscirpendiol
- Fusarenone X
- Verrucarin A, B, J
- Roridin A, D, E, H
- Many Others (29+)
These are “field” toxins, not “storage” toxins
Other Mycotoxins of Growing Interest
- Ochratoxins
- Produced by Penicillium verrucosum and several spp. of Asperfillus.
- Potently nephrotoxic and carcinogenic, teratogenic and immunotoxic.
- Public health problem, but little evidence of problematic instances in swine.
Other Common Molds
- Penicillium
- Common blue mold
- Capable of producing mycotoxin, usually does not.
- Affected cattle and sheep in Africa
- Diplodia
- Fusarium
- Taxonomy is quite confusing
- Has had classification changed various times
- Fusarium roseum, Fusarium graminearum and Gibberella zeae are all terms applied to the same thing.
- Gibberella zeae is the “perfect” (reproductive) stage
- Nickname “GIB” corn.
- Fusarium toxins
- Deoxynivalenol
- Feed refusal
- Emesis (so nicknamed “vomitoxin”)
- Zearalenone
- Estrogenic effects
Symptoms of Mycotoxicosis:
1. Drugs and antibiotics are not effective in treatment.
2. The symptoms can be traced to foodstuffs or feed.
3. Testing of said foodstuffs or feed reveals fungal contamination.
4. The symptoms are not transmissible person to person.
5. The degree of toxicity is subject to persons age (more often in very young and very old), sex
(more often in females than males) and nutritional status.
6. Outbreaks of symptoms appear seasonally
Regulatory Control:
- In 1965, the Food and Drug Administration (FDA) set the first mycotoxin limit of 20 parts per billion (ppb) for aflatoxin in all foods and feed.
- But, this toxin can appear at varying levels of food production, so multiple testing at different points in the food chain is necessary.
- Using ELISA (enzyme-linked immunosorbent assay) technology, testing can be done cheaper and faster than previously.
- The FDA does not do the testing, various other agencies do, such as the Grain Inspection Packers and Stockyards Administration; but, toxic levels must be reported to the FDA.
Fusarium
- Fungal species from the genus Fusarium will attack corn and wheat plants.
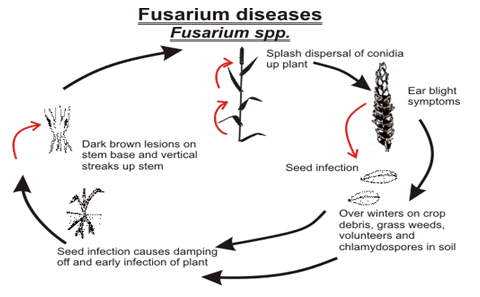
- Most are plant pathogens and can be found in soil.
- Some examples of affected plants are corn, wheat, barley, beans, with lesser contamination in rye, triticale, millet, and oats.
- Trichothecene toxins target the circulatory, alimentary, skin, and nervous systems.
F. graminearum- Wheat
- Causes scab damage to kernels and head blight.
- Produces deoxynivalenol (DON), also called vomitotoxin.
F. graminearum in Maize (Corn)
- Creates Giberella Ear Rot
- Produces the toxins: DON and zearalenone (ZEN), and T-2 toxins.
- Have damaging effects on plants, humans, and other animals with monogastric digestive processes
DON and T-2 Toxin:
- These are tricothecenes of wheat, grain, and barley.
- They cause necrosis and hemorrhage of the digestive tract, decreased blood production in the bone and spleen, and changes to reproductive systems.
- In poultry, causes reduced egg production, beak lesions, and abnormal feathering
- Optimal temperature range is between 70 and 85 0 F
- Advisory level of DON is 1 ppm.
Zearalenone:
- A tricothecene.
- Mimics the body’s production of estrogen.
- Causes feminization of male animals.
- Disrupts conception, ovulation, and fetal development in female animals.
- Pigs are especially sensitive, poultry and cows show little sensitivity.
Zearalenone often occurs with DON in naturally-contaminated cereals. Zearalenone is responsible for reproductive disorders due to its oestrogenic effect at high concentrations. However, in general ZEA has limited toxicity to birds.
At high concentrations the following symptoms have been observed:
- Vent enlargement
- Enhanced secondary sex characteristics
F. moniliforme
- Plant pathogen most associated with corn. Also found in rice, sorghum, yams, hazelnuts, pecans, and cheeses.
- Diseases associated with this species include “crazy horse disease” in horses, pulmonary edema in pigs, liver cancer in rats, bone malformation in chicks and pigs.
- The fumonisins produced by F. moniliforme are linked with esophageal cancer in humans.
- Other toxins produced include fusaric acid, fusarins, and fusariocins.
- Advisory levels are 5 ppm in animal feed.
Alternaria Toxins
- Infects the plant in the field, such as wheat, sorghum, and barley.
- Also fruits and vegetables that can cause spoilage in refrigeration.
- Toxins include: alternariol, alternariol monomethyl ether, altenuene, tenuazonic acid, and altertoxins.
- Little is know of these toxins; but, toxic effects are seen in rats, chicks, ducklings, and turkeys.
Claviceps Toxins
- Earliest recognized mycotoxicosis caused by C. purpurea, with ergot mold.
- Outbreaks have been reported since 857 A.D.
- The Middle Ages had near epidemic proportions.
- Humans consumed bread baked with grain containing ergot spores, which produced lysergic acid diethylamide (LSD) symptoms and hallucinations.
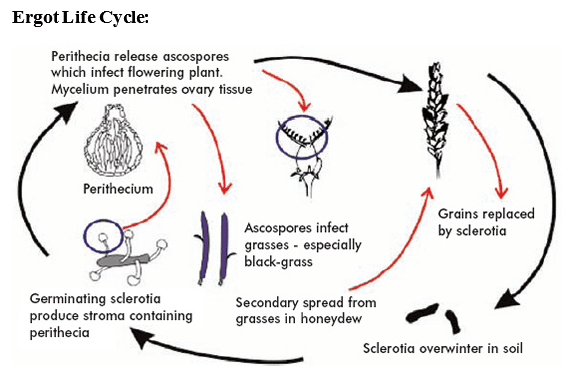
Ergot Poisoning:
- Disease of barley, oats and wheat.
- Grains are replaced by ergot sclerotia that contain toxins.
- Main toxin is called ergotamine.
- Named for the belief that a pilgrimage to the shrine of St. Anthony would alleviate the symptoms.
Symptoms:
- Dry gangrene,
- Internal bleeding,
- Vomiting,
- Constipation,
- Diarrhea
Aspergillus Toxins:
- Large genus with more than 100 species, 50 of which are known to produce mycotoxins.
- Some of which are aflatoxin, ochratoxin A, sterignmatocystin, cycolopiazonic acid, citrinin, patulin, and tremorgenic toxin.
- Aspergillusniger is used to make artificial citric acid; one use is in soft drinks.
- Miso, soy sauce, and sake use strains of A. oryzae.
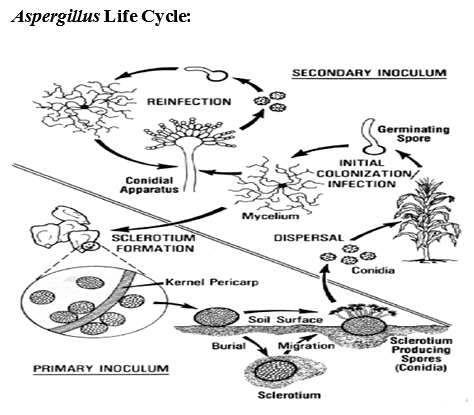
Aspergillus and Aflatoxin:
- Aflatoxicosis: caused by high doses in short intervals or low doses in high intervals.
- 1961, caused the deaths of over 100,000 turkey poults: “Turkey X disease”.
- Toxin was traced to contaminated Brazilian peanut meal in poultry feed.
- Grows best between 80-90 0 F
- Aflatoxins are of concern in warm and humid climatic conditions.
- Damage to grain increases likelihood of fungal growth
Aflatoxin B-1:
- Definite link to cancer in animals.
- Possible link to cancer in humans. Studies done in Africa and Asia show a correlative link, but not a causative one.
- Primarily attacks the liver, in cases of cirrhosis, necrosis, and carcinomas with a secondary affect of immune suppression.
- Risk factor for neonatal jaundice, in areas of maternal consumption.
- Does not stay in the body for long periods of time, usually excreted within 96 hours, in animals.
- In milk, for human consumption, advisory level is 5 ppb.
Among poultry, ducks are the most susceptible to aflatoxin, followed by turkeys, broilers, laying hens and quail. In all species, aflatoxins are hepathotoxic with fatty changes, causing hepathocyte degeneration, necrosis, and altered liver function. Suppression of hepatic protein synthesis is the main factor resulting in growth suppression and reduced egg production. Aflatoxin is also known to interfere with vitamin D metabolism, contributing to reduced bone strength and leg weakness. By reducing bile salt production, aflatoxin negatively affects lipid and pigment absorption. Additionally, the metabolism of other minerals including iron, phosphorus and copper are also affected by aflatoxin. Aflatoxin increases the fragility of capillaries, reducing prothrombin levels thereby drastically increasing the incidence of bruising in carcasses and carcass downgrading. Due to the transfer of aflatoxin into edible products and its carcinogenic effects, most countries have set upper legal limits for aflatoxin in feed
Clinical signs of Aflatoxin Toxicity:
- Embryo toxicity
- Specific visceral haemorrhage
- Increased susceptibility to environmental and microbial stressors
- Leg weakness and reduced bone strength
- 'Pale bird syndrome'
- Fatty liver
- Liver necrosis
- Bile duct hyperplasia
- Increased incidence of bruising and downgrading

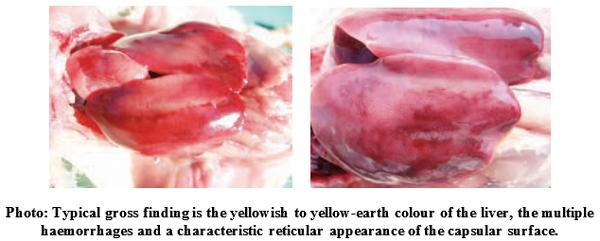
Lesions:
- An increase in the size of the liver, kidneys and spleen,
- Liver becoming friable, pale in colour and extremely fatty with increasing severity of the outbreak.
- Lymphoid tissue, including the bursa of Fabricius and thymus glands, undergo astrophy.
- The gall bladder is usually enlarged and filled with bile that appears dilute.
- An increased susceptibility to bruising, resulting from increased capillary fragility, is often observed.
- This effect is manifested by an increased incidence of Petechial haemorrhages of the musculature
- Decreased egg production and diminished interior and exterior egg quality is typical.
- In breeders, increased embryonic mortality, decreased hatchability and an increased incidence of malformed embryos will be evident.
- These effects occur from the transfer of aflatoxin or its metabolites to the egg during the time of dietary intake of toxin.
- Within 7 days after the cessation of aflatoxin feeding, marked improvement in hatchability can be observed
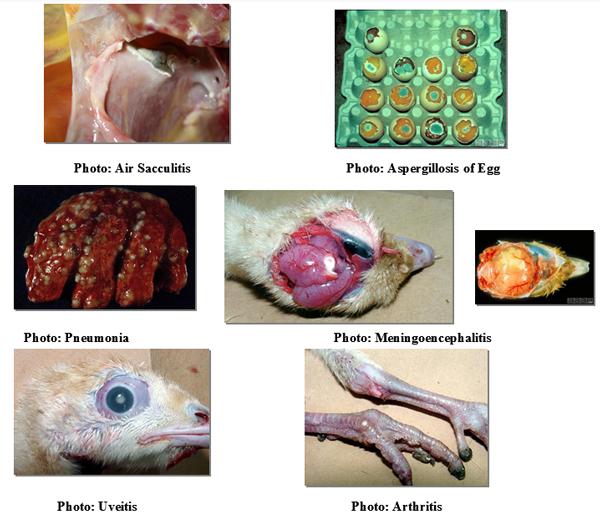
Prevention and Control:
Use clean eggs free of cracks and fresh litter free of hard wood, dusty rice hulls and peanut hulls. Maintain a clean environment in the hatchery and during brooding.
Treatment:
Treatment is very difficult as soon as the infection has been established, although enilconazole, sprayed in the house, is known to aid in preventing new infection. There is a regulatory issue here, as disinfection of the environment in presence of animals could be illegal in European conditions. Several disinfectants are used for prevention, but care should be given to the claimed activity and safety in use. Several producers claim a sporocidal activity, but when taking a closer look at the activity, the sporocidal activity is only evident after a very long contact time and/or high concentration. Fungal spores are extremely difficult to destroy by disinfection. Some of these compounds work on the metabolism, so when spores are not sporulating, they cannot exert their activity. When these compounds are washed away from the spores, they will be able to sporulate again. These compounds should be called anti-sporulant instead of sporocidal.
Penicillium Toxins:
- Large genus with over 150 species.
- Discovered antibacterial properties within genus, causing production of penicillin.
- 100 species have mycotoxins.
- Nine specific toxins affecting human health are citreoviridin, citrinin, cyclopiazonic acid, ochratoxin A, patulin, penitrem A, PR toxin, Roquefortine C, and, Secalonic acid D.
- Separated into two groups: those that affect liver and kidneys, and those that are neurotoxic.
- Liver and kidney toxins are asymptomatic and cause overall animal debility.
- Neurotoxins cause visible trembling.
Ochratoxin A and Citrinin:
- Affects kidney function.
- Causes Balkan nephropathy and Yellow Rice Fever in humans.
- Chickens, turkeys, and ducklings are affected by ochratoxicosis, causing poor weight gain, egg output, and poor shell quality.
- Ochratoxin sources are peanuts, pecans, beans, dried fruit and dried fish.
- Citrinin sources are in wheat, rice, corn, and flour.
- Citrinin is most associated with horses, pigs, dogs, and poultry.
Ochratoxin:
Ochratoxins are important storage toxins. They are produced by different fungi and are prevalent in temperate as well as in tropical regions.
Ochratoxin A is the most important of the ochratoxins. The primary effect of ochratoxin A in all poultry species is nephrotoxicity. In poultry, the proximal tubules are mainly affected and the kidney is pale and grossly enlarged. As with aflatoxin, fatty liver can also occur due to ochratoxin exposure. In acute cases, mortalities can occur due to acute renal failure. In young chicks, ochratoxin A is approximately three times more toxic than aflatoxin.
Ochratoxin A is the most important of the ochratoxins. The primary effect of ochratoxin A in all poultry species is nephrotoxicity. In poultry, the proximal tubules are mainly affected and the kidney is pale and grossly enlarged. As with aflatoxin, fatty liver can also occur due to ochratoxin exposure. In acute cases, mortalities can occur due to acute renal failure. In young chicks, ochratoxin A is approximately three times more toxic than aflatoxin.
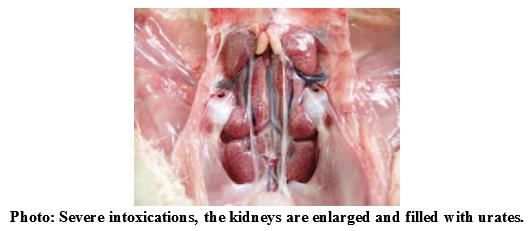
Clinical signs of ochratoxin toxicity include:
- Mortality due to acute renal failure
- Poor eggshell quality and higher incidence of eggs with blood spots
- Reduced embryo viability and decreased hatchability
- Reduced feathering
- Polyurea with large volumes of wet faeces
- Pale and grossly enlarged kidney
- Fatty liver
- Urate deposition in joints and abdominal cavity (at high exposure levels)
Depletion of lymphocytes and with it strong suppression of cellular immunity, thus enhanced susceptibility to viral infections
Cyclopiazonic Acid (CPA):
- Found in corn and peanuts in Georgia.
- Chief species from Penicillium causes cheese spoilage.
- Causes fatty degeneration in liver and kidneys in animals, chickens are very susceptible.
- May act synergistically with aflatoxin.
Future Fight against Mycotoxins:
- Have farmers select strains resistant to contamination.
- Scientists hope to genetically engineer plants resistant to fungal infection.
- Use feed additives that sequester the toxins and prevent absorption from the gastrointestinal tract
Poultry
Economic losses associated with mycotoxicosis include:
- Poor growth
- Reduced egg production
- Reduced feed conversion
- Increased mortality
- Poor egg shell quality
- Reduced fertility
- Leg problems
- Carcass condemnation
- Increased susceptibility to disease
Fumonisin:
Broilers relatively resistant to acute toxic effects of fumonisins. Fumonisin mycotoxicosis leads to a very specific increase in sphiganine:sphingosine ratio. However, as sphinganine and sphingosine analyses are quite complex this ratio is seldom used as a biomarker in field situations.
Clinical signs of fumonisin toxicity include:
Clinical signs of fumonisin toxicity include:
- Spiking mortality (paralysis, extended legs and neck, wobbly gait, gasping)
- Reduced growth rate
- Increased organ weights
- Hepatocellular hyperplasia
- Poor vaccination response
- Increased liver sphinganine: sphingosine ration (biomarker)
Feeding Strategies:
As the risk, if mycotoxicosis is very difficult to predict or evaluate, prevention strategies should be initiated when assessing even a low-risk situation. Prevention strategies must primarily aim at minimising mycotoxin formation in the field and during storage.
A significant reduction in mycotoxin formation can be achieved by good agronomic practices, for example:
A significant reduction in mycotoxin formation can be achieved by good agronomic practices, for example:
- Selection of crop varieties that are more resistant to fungal foliar diseases
- Ploughing up harvest residues
- Avoiding no-till soil management practices
- Proper crop rotation
- Avoiding monoculture
During storage of dry feed ingredients, mycotoxin formation can be successfully controlled by monitoring the moisture content of the feed. If the moisture content is below 12%, moulds become metabolically inactive, and the risk of mycotoxin formation is strongly reduced. To avoid mycotoxin formation, be aware of the following:
- Moisture content below 12%
- Relative humidity below 60%
- Storage temperature below 20 °C
- Clear grain, avoid broken kernels
- Control insects and rodents
- Avoid stress (frost, heat, pH changes)
Mycotoxin adsorbents and binders:
As we know mycotoxins are usually found in combinations in complete animal feeds. A broad substrate binding capacity will ensure at least some fraction of all the mycotoxins will be rendered non-bioavailable and the bioavailable mycotoxins will be below the threshold of biological activity. Broad substrate binding capacity of a binding agent will also minimise the potential for toxicological synergy between mycotoxins.
Speciality feed additives, known as mycotoxin adsorbents or binding agents are the most common approach to prevent and treat mycotoxicosis in animals. It is believed that the agents bind to the mycotoxin preventing them from being absorbed. The mycotoxins and the binding agent are excreted in the manure.
The effective level of dietary inclusion for mycotoxin adsorbents will depend on the mycotoxin binding capacity of the adsorbent and the degree of contamination of the feed in question. A high binding capacity will minimise the level of inclusion and minimise the reduction in nutrient density caused by the feeding of the adsorbent. High levels of inclusion of adsorbents can also alter the physical properties of the feed which might impair feed processing such as pellet formation, in addition to altering the actual diet specification.
Mycotoxin binding is achieved through both:
- Physical adsorption
- Relatively weak bonding involving van der Waals interactions and hydrogen bonding
- Chemical Adsorption: (Chemisorption) is a stronger interaction which involves ionic or covalent bonding.
An effective binder or sequestering agent is one that prevents or limits mycotoxin absorption from the gastro-intestinal tract of the animal. In addition, they should be free from impurities and odours. Be aware that not all are equally effective. Many can impair nutrient utilisation and are mainly marketed, based on in-vitro data only.
There are two types of mycotoxin adsorbent/binder:
- Inorganic binders
- Organic adsorbents
Inorganic binders:
Inorganic mycotoxin binders are silica-based polymers. Examples could include:
Inorganic mycotoxin binders are silica-based polymers. Examples could include:
- Zeolites
- Bentonites
- Bleaching clays from the refining of canola oil
- Hydrated sodium calcium aluminosilicates (HSCAS)
- Diatomaceous earth
- Numerous clays
They can be grouped into two categories: Phyllosilicates and Tectosilicates
Phyllosilicates: bentonites/montmorillonites :
Phyllosilicates: bentonites/montmorillonites :
- Phyllosilicates are characterised by alternating layers of tetrahedral silicon and octahedral aluminium coordinated with montmorillonite oxygen atoms
- Isomorphous substitution leads to a net negative charge which must be satisfied by the presence of inorganic cations (Na, Ca, Mg, K)
- Applications: Adsorbents for heavy metals, suspension-stabilising agents in coatings, bonding agents for foundry sands and washes, binder in pelletisation processes, desiccants in feed products.
Tectosilicates: Zeolites :
- Tectoalumosilicates of alkali and alkaline earth cations that have an infinite three-dimensional cage-like structure
- Isomorphous substitution leads to a net negative charge which is satisfied by the presence of inorganic cations (Na, Ca, Mg, K)
- Applications: Adsorbents for ammonia, heavy metals, radioactive cesium and mycotoxins.
Organic Adsorbents:
Organic mycotoxin adsorbents are carbon based polymers. Examples could include:
Fibrous plant sources such as:
- Oat hulls
- Wheat bran
- Alfalfa fibre
- Extracts of yeast cell wall
- Cellulose
- Hemi-cellulose
- Pectin
Such materials are biodegradable but can, in some cases, also be vectors of mycotoxin contamination. Benefits of yeast cell wall are low inclusion, high surface area and certainly no toxic contaminants. The efficacy of glucomannan-containing yeast products as mycotoxin adsorbents in feeds has been investigated globally with several studies with all animals
Mycotoxin adsorbents offer an attractive short-term solution to the challenge of mycotoxin-contaminated animal feeds. The only complete solution to the mycotoxin challenge will be the long-term goal of eliminating mycotoxins from the food and feed chains through improved quality control based on better analytical techniques coupled with genetic advances in plant resistance to fungal infestation.
Mycotoxin adsorbent should be:
Mycotoxin adsorbents offer an attractive short-term solution to the challenge of mycotoxin-contaminated animal feeds. The only complete solution to the mycotoxin challenge will be the long-term goal of eliminating mycotoxins from the food and feed chains through improved quality control based on better analytical techniques coupled with genetic advances in plant resistance to fungal infestation.
Mycotoxin adsorbent should be:
- Proven efficacy in vivo as well as in vitro
- Low effective inclusion rate
- Stable over a wide pH range (This is necessary so that the mycotoxin stays attached to the adsorbent throughout the gut and is excreted.)
- High affinity to adsorb low concentrations of mycotoxins
- High capacity to adsorb high concentrations of mycotoxins
- Ability to act rapidly before the mycotoxin can be absorbed into the bloodstream.
Related topics:
Authors:
Suman Hatchery Ltd.
Recommend
Comment
Share

27 de marzo de 2020
In total agreement with the conclusion of the author on the need to use both short- and long term approahces to minimize mycotoxins in food/feed value chain.
Recommend
Reply

Would you like to discuss another topic? Create a new post to engage with experts in the community.





