Evaluation of Sperm Parameters, Reproductive Hormones, Histological Criteria, and Testicular Spermatogenesis Using Turnip Leaf (Brassica Rapa) Hydroalcoholic Extract in Male Rats: An Experimental Study
Turnip leaves are rich in vitamins, contain large amounts of various substances with biological properties, and contain various flavonoids, some of which have phytoestrogens properties. The effect of hydroalcoholic extract of turnip leaf (TLE) on pituitary-gonad axis and testicular tissue changes in adult male rats was investigated. Forty Wistar rats were used in 5 groups of 8. The control group used water and standard laboratory feed and did not receive any TLE. The placebo group received orally a certain amount of distilled water as an extracting solvent. Experimental groups 1, 2, and 3 used TLE included 500, 1000, and 2000 mg/kg, respectively, for 28 days. Finally, blood samples were collected from all examined groups to measure the serum concentrations of testosterone, LH, and FSH by the ELISA method. The testes were removed from the animals, and the diameter of the seminiferous tubules (STD) was measured by DinoCapture software. The results revealed that BW, left and right testes did not exhibit significant changes. The results of hormonal tests showed that the TLE in experimental groups increased the level of testosterone and had no significant effect on the levels of LH and FSH. Histological studies showed that the number of spermatogonia, spermatocytes, spermatids, spermatozoa, Leydig cells, STD, and germinal epithelium diameter (GED) in experimental groups showed significance. The effects of TLE were not dose-dependent and the value of 1000 mg/kg is recommended for effectiveness and margin of assurance. This research article is taken from the master's thesis in the field of animal biology.
Keywords: Plant extract, Phytoestrogens, Spermatogenesis, Testosterone, Histomorphometry.
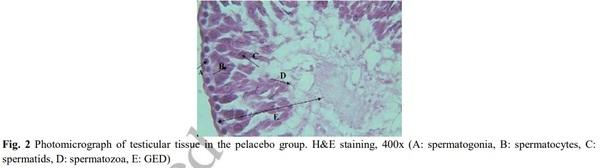
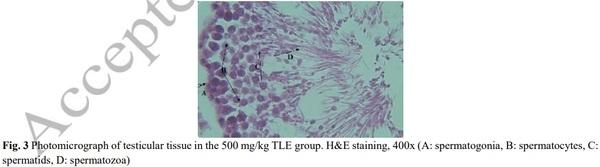
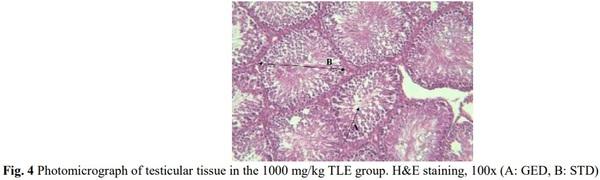
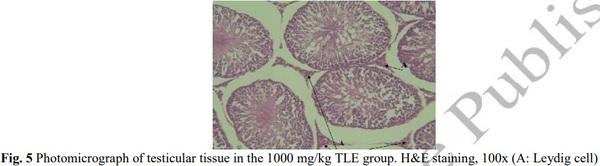
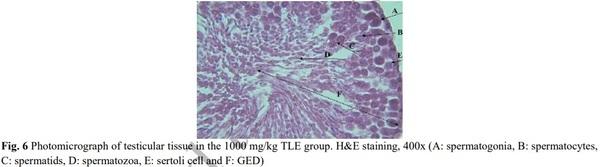
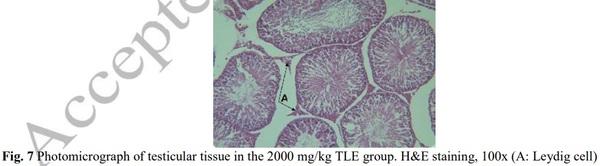
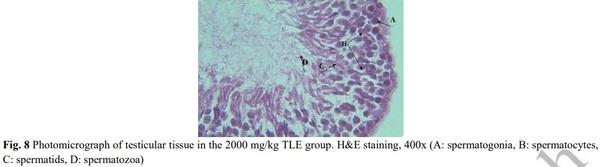
[1] Geelhoed DW, Nayembil D, Asare K, Van Leeuwen JS, Van Roosmalen J. Infertility in rural Ghana. International Journal of Gynecology & Obstetrics. 2002; 79(2): 137-142.
[2] Kumar N, Singh AK. Trends of male factor infertility, an important cause of infertility: A review of literature. Journal of human reproductive sciences. 2015; 8(4): 191.
[3] Cramer DW, Walker AM, Schiff I. Statistical methods in evaluating the outcome of infertility therapy. Fertility and Sterility. 1979; 32(1): 80-86.
[4] Ghuman N, Ramalingam M. Male infertility. Obstetrics, Gynaecology & Reproductive Medicine. 2018; 28(1): 7-14.
[5] Brentani H. Gender, Genetic, And Environmental Factors In The Neurodevelopmental Disorders. European
Neuropsychopharmacology. 2019; 29: 745-746.
[6] Sekhon V, Luthra M, Jevalikar G. Persistent Mullerian Duct Syndrome presenting as irreducible inguinal hernia–A surprise surgical finding!. Journal of Pediatric Surgery Case Reports. 2017; 16: 34-36.
[7] Trama A, Foschi R, Larrañaga N, Sant M, Fuentes-Raspall R, Serraino D, Tavilla A, Van Eycken L, Nicolai N, Hackl M,
Bielska-Lasota M. Survival of male genital cancers (prostate, testis and penis) in Europe 1999–2007: Results from the EUROCARE- 5 study. European Journal of Cancer. 2015; 51(15): 2206-2216.
[8] Chen L, Shi GR, Huang DD, Li Y, Ma CC, Shi M, Bin-xiao Su, Shi G J. Male sexual dysfunction: a review of literature on its pathological mechanisms, potential risk factors, and herbal drug intervention. Biomedicine & Pharmacotherapy. 2019; 112: 1-13. [9] Saleh RA, Agarwal A, Sharma RK, Nelson DR, Thomas Jr AJ. Effect of cigarette smoking on levels of seminal oxidative stress in infertile men: a prospective study. Fertility and sterility. 2002; 78(3): 491-499
[10] Tolstrup JS, Kjær SK, Holst C, Sharif H, Munk C, Schmidt L, Andersen AMN, GrØnbÆk M. Alcohol use as predictor for infertility in a representative population of Danish women. Acta obstetricia et gynecologica Scandinavica. 2003; 82(8): 744-749.
[11] Lundy SD, Sabanegh Jr ES. Varicocele management for infertility and pain: a systematic review. Arab journal of urology. 2018; 16(1): 157-170.
[12] Comhaire F, Vermeulen L. Human semen analysis. Human reproduction update. 1995; 1(4): 343-362.
[13] Hao DC, Xiao PG. Pharmaceutical resource discovery from traditional medicinal plants: Pharmacophylogeny and pharmacophylogenomics. Chinese Herbal Medicines. 2020; 12(2): 104-117.
[14] Ishimi Y, Takebayashi J, Tousen Y, Yamauchi J, Fuchino H, Kawano T, Inui T, Yoshimatsu K, Kawahara N. Quality evaluation of health foods containing licorice in the Japanese Market. Toxicology reports. 2019; 6: 904-913.
[15] Javed A, Ahmad A, Nouman M, Hameed A, Tahir A, Shabbir U. Turnip (Brassica Rapus L.): a natural health tonic. Brazilian
Journal of Food Technology. 2019; 22:1-9.
[16] Nabeshima EH, Moro TM, Campelo PH, Sant'Ana AS, Clerici MTP. Tubers and roots as a source of prebiotic fibers.
Probiotic and Prebiotics in Foods: Challenges, Innovations and Advances. 2020; 94:267-293.
[17] Tridge. https://www.tridge.com/intelligences/turnip/production 2019.
[18] Fernandes F, Valentão P, Sousa C, Pereira JA, Seabra RM, Andrade PB. Chemical and antioxidative assessment of dietary turnip (Brassica rapa var. rapa L.). Food Chemistry. 2007; 105(3): 1003-1010.
[19] Bennetau-Pelissero C. Natural Estrogenic Substances, Origins, and Effects, In: Mérillon JM., Ramawat K. (eds) Bioactive
Molecules in Food. Reference Series in Phytochemistry. Springer, Cham. 2018; 1-70. [20] Stahl S, Chun TY, Gray WG. Phytoestrogens act as estrogen agonists in an estrogen-responsive pituitary cell line. Toxicology and Applied Pharmacology. 1998; 152(1): 41-48.
[21] Patisaul HB, Jefferson W. The pros and cons of phytoestrogens. Frontiers in neuroendocrinology. 2010; 31(4): 400-419.
[22] Baumann J, Bruchhausen FV, Wurm G. Flavonoids and related compounds as inhibitors of arachidonic acid peroxidation.
Prostaglandins. 1980; 20(4): 627-639.
[23] You KM, Jong HG, Kim HP. Inhibition of cyclooxygenase/lipoxygenase from human platelets by polyhydroxylated/methoxylated flavonoids isolated from medicinal plants. Archives of pharmacal research. 1999; 22(1): 18-24.
[24] Zerani M, Catone G, Quassinti L, Maccari E, Bramucci M, Gobbetti A, Maranesi M, Boiti C, Parillo F. In vitro effects of gonadotropin-releasing hormone (GnRH) on Leydig cells of adult alpaca (Lama pacos) testis: GnRH receptor immunolocalization, testosterone and prostaglandin synthesis, and cyclooxygenase activities. Domestic animal endocrinology. 2011; 40(1):51-59.
[25] Grippo AA, Capps K, Rougeau B, Gurley BJ. Analysis of flavonoid phytoestrogens in botanical and ephedra-containing dietary supplements. Annals of Pharmacotherapy. 2007; 41(9): 1375-1382.
[26] Parvin Jahromi S, Zahiri S, Mahjoor AA, Safarpour D. Histopathological and stereological studies of soybean hydroalcoholic extract effects on the rat ovary. ISMJ. 2012; 15(3): 161-170.
[27] Clavijo RI, Hsiao W. Update on male reproductive endocrinology. Translational andrology and urology. 2018; 7(Suppl 3),
S367.
[28] Ganong WF. Review of Medical Physiology, 22. USA: Appleton and Lange; 2005. 928 pp.
[29] Willems E, Knigge U, Jørgensen H, Kjær A, Warberg J. Effect of selective blockade of catecholaminergic alpha and beta receptors on histamine-induced release of corticotropin and prolactin. Neuroendocrinology. 1999; 69(5): 309-315.
[30] Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiological reviews. 2002; 82(4): 825-874.
[31] Boron WF, Boulpaep EL. Mammalian Physiology Organization of the Endocrine Sysyem. Medical Physiology. 2004; 345- 400.
[32] Courot M, Ortavant R. Endocrine control of spermatogenesis in the ram. J Reprod Fertil Suppl. 1981; 30: 47-60.
[33] McLachlan RI. The endocrine control of spermatogenesis. Best Practice & Research Clinical Endocrinology & Metabolism. 2000; 14(3): 345-362.
[34] Lin JX, Jia YD, Zhang CQ. Effect of epidermal growth factor on follicle-stimulating hormone-induced proliferation of granulosa cells from chicken prehierarchical follicles. Journal of Zhejiang University SCIENCE B. 2011; 12(11): 875-883.
[35] Wong RWC, Kwan RWP, Mak PHS, Mak KKL, Sham MH, Chan SY. Overexpression of epidermal growth factor induced hypospermatogenesis in transgenic mice. Journal of Biological Chemistry. 2000; 275(24): 18297-18301.
[36] Spiteri-Grech J, Bartlett JMS, Nieschlag E. Regulation of testicular insulin-like growth factor-I in pubertal growth hormone- deficient male rats. Journal of endocrinology. 1991; 131(2): 279-285.
[37] Cattoni A, Clarke E, Albanese A. The predictive value of insulin-like growth factor 1 in irradiation-dependent growth hormone deficiency in childhood cancer survivors. Hormone research in paediatrics. 2018; 90(5): 314-325.
[38] Cannarella R, Mancuso F, Condorelli RA, Arato I, Mongioì LM, Giacone F, Lilli C, Bellucci C, La Vignera S, Calafiore R,
Luca G. Effects of GH and IGF1 on basal and FSH-modulated porcine sertoli cells in-vitro. Journal of clinical medicine. 2019; 8(6):
811. [39] Norman JF, Way JM. Some ecological observation on the use of date pollen in hypothalamic hormones. Endocrinol. Journal of chemical Ecology, ogy. 1998; 2: 532-548.
[40] Walker LE. The Importance of Environmental Embodiment for Public Health Professionals: Stress Triggers, Environmental
Toxicants, and Strategies for Education (Doctoral dissertation), 2015; 129 pp. [41] Ge R, Chen G, Hardy MP. The role of the Leydig cell in spermatogenic function. Molecular Mechanisms in Spermatogenesis. 2009; 255-269.
[42] Hansson V, Weddington SC, McLean WS, Smith AA, Nayfeh SN, French FS, Ritzen EM. Regulation of seminiferous tubular function by FSH and androgen. Reproduction 1975; 44(2): 363-375.
[43] Pietta PG. Flavonoids as antioxidants. Journal of natural products. 2000; 63(7): 1035-1042.
[44] Tarin JJ, Vendrell FJ, Ten J, Cano A. Antioxidant therapy counteracts the disturbing effects of diamide and maternal ageing on meiotic division and chromosomal segregation in mouse oocytes. Molecular human reproduction. 1998; 4(3): 281-288.
[45] Aslani F, Sebastian T, Keidel M, Fröhlich S, Elsässer HP, Schuppe HC, Klug J, Mahavadi P, Fijak M, Bergmann M,
Meinhardt A. Resistance to apoptosis and autophagy leads to enhanced survival in Sertoli cells. MHR: Basic science of reproductive medicine. 2017; 23(6): 370-380.
[46] O'Donnell L, Stanton P, de Kretser DM. Endocrinology of the Male Reproductive System and Spermatogenesis. In: De Groot
LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM et al., editors. Endotext. South Dartmouth (MA). 2000.
[47] Tripathi UK, Chhillar S, Kumaresan A, Aslam MM, Rajak SK, Nayak S, Manimaran A, Mohanty TK, Yadav S.
Morphometric evaluation of seminiferous tubule and proportionate numerical analysis of Sertoli and spermatogenic cells indicate differences between crossbred and purebred bulls. Veterinary world. 2015; 8(5): 645-50.








.jpg&w=3840&q=75)