In the most simplistic terms, the body (humans and animals) could be compared to a medium in which millions of chemical reactions take place with the support of food energy. These reactions are the basis of life without which life will cease to exist. The body can simply be called the “fire of life” because oxygen is one of the main substances required by humans and animals to maintain this fire. This fire can neither be allowed to burn out of control nor be extinguished completely. Therefore, for a healthy bodily function, an optimum burning of this fire is necessary.
Oxidative stress occurs when the generation of reactive metabolites of oxygen (reactive oxygen species or free radicals) exceeds their safe detoxification or disposal by the body. In animal production, reactive metabolites of oxygen may occur as a result of physical, biological, and chemical stressors (stress of production) which could result in morbidity, mortality or reduced production. Free radicals include singlet oxygen, superoxide, hydrogen peroxide, hydroxyl radical and fatty acid radicals. Free radicals can cause damage or even cause cell death (process of ageing) when they react with enzymes, cell membranes and DNA. Cellular defenses to control or neutralize the harmful effects of free radicals in the body are through
1) Enzymatic neutralizing defenses (Zn/Cu/Mn superoxide dismutase, Fe-catalase, Se-glutathione peroxidase and Se-glutathione-S-transferase),
2) Enzymatic damage repair defenses (lipases, proteases and DNA/RNA repair enzymes),
3) Non-enzymatic defenses (glutathione, uric acid, melatonin, hypotaurine) and
4) Nutrient defenses (carotenoids, ascorbate, tocopherols, tocotrienols, phenols, lycopenes, trace minerals, etc.).
In animal production, the most efficient approach to controlling oxidative stress is through nutrient defenses or antioxidant supplementation. In dairy cows, some important health disorders (retained fetal membranes, udder edema, mastitis) appear to be related to oxidative stress. Also, milk quality and shelf-life could be affected by reduced antioxidants such as vitamins A, C and E. Pre- and post-weaning periods are very stressful for dairy calves because of the changes in diet and biological, chemical and environmental stressors. The resultant effect is a depressed immune function predisposing them to infection by deadly pathogens. Premature development of the rumen in these calves often results in reduced vitamin C synthesis, precipitating a reduction in total antioxidant capacity. In such conditions where vitamin C is depleted, supplementation would be beneficial. The challenge would be to how to supplement a stabilized source of vitamin C to these animals.
Do multiple stomach animals have a requirement for vitamin C?
Research on swine and poultry (7, 8) suggests that under specific environmental and physiological conditions the amount of ascorbic acid produced by an animal may be insufficient to meet its requirements. Lactating dairy cows may be predisposed to subclinical ascorbic acid deficiency because of the drain on the precursor molecules for ascorbic acid production, glucose and galactose into milk. Non-lactating ruminants, especially calves, may also have their ascorbic acid status compromised during periods of stress (5). When calves were subjected to environmental stressors the supplementation of ascorbic acid has been shown to enhance their immune response (4). The physiological ramifications of subclinical ascorbic acid deficiency are multifactorial. Ascorbic acid is critical if the effectiveness of the immune system is to be optimized (2, 3). Pathogenic organisms such as bacteria and viruses cause increased free radical production in the infected host and a subsequent decrease in ascorbic acid level and immune function (1). The need for post ruminal vitamin C was long known from the infusion of this nutrient into the lower gut of ruminants. The challenge has always been how to find a practical solution to deliver vitamin C into the lower gut of ruminants. Ascorbic acid in its crystalline state is extremely unstable in the conditions which exist in the rumen (6). Unprotected and non-microencapsulated vitamin C supplements are exposed to temperature, pH, and air destruction before they are needed which render them unstable. Typically, overdosing is done to compensate for the stability losses. Even with overdosing, adequate amounts of vitamin C do not flow to the lower gut where the nutrient can be utilized. On the other hand, stabilized microencapsulated vitamin C, when used as a supplement in animal feed, is temperature and low pH resistant, is protected from ruminal microbial degradation and becomes biologically available to all ruminants and nonruminants as long as it reaches the lower gut.
Research Study
A study was conducted at The F.A.R.M.E Institute in NY to evaluate the stability of two activities (75%, and 50%) of microencapsulated vitamin C in the rumen of cows using in situ procedures and ruminally cannulated dairy cows. A secondary objective was to determine the effect of particle size distribution on rumen bypass of the three activities of microencapsulated vitamin C. The particle of each activity of Vitamin C was enrobed with a food grade GRAS vegetable matter. This means that for the 75%, and 50%, the coating amounts were 25% and 50%, respectively.
Procedures
Samples were tested for digestibility in situ using high producing lactating dairy cows. Samples were run in a single cannulated cow. Cows used in this test were in mid-lactation. The cows were fed a standard dairy cattle ration balanced according to 2001 National Research Council (NRC) recommendations. Each sample was run in triplicate with 5 grams of dry matter (DM, approximate) in each subset.
In situ testing involved weighing samples, in the form they were received, into a 10cm x 20cm nitrogen-free polyester bag with a pore size of 50 (+/- 15) microns. The bags were heat sealed. Samples were soaked prior to incubation in the rumen of a cannulated cow. Ruminal incubation lasted 16 hours to simulate expected retention times for products of this type. A second, 48 hour incubation was conducted to estimate total ruminal digestibility.
After ruminal incubation, samples were frozen and then machine rinsed with cold water. Samples were then fully dried in a convection drying oven (minimum of 24 hours at 120 degrees F) and weighed. For the microencapsulated vitamin C samples and that of the coating, each in situ bag was placed in a weigh boat to dry. This prevented dry matter loss due to lipid approaching the melting point.
All weights were recorded into a spreadsheet file. Standard disappearance calculations were used and coefficients of variation determined for each sample. Outliers were not used in the final average if coefficient of variations (CV’s) were greater than 5%. In this study, only dry matter disappearance was determined.
Results
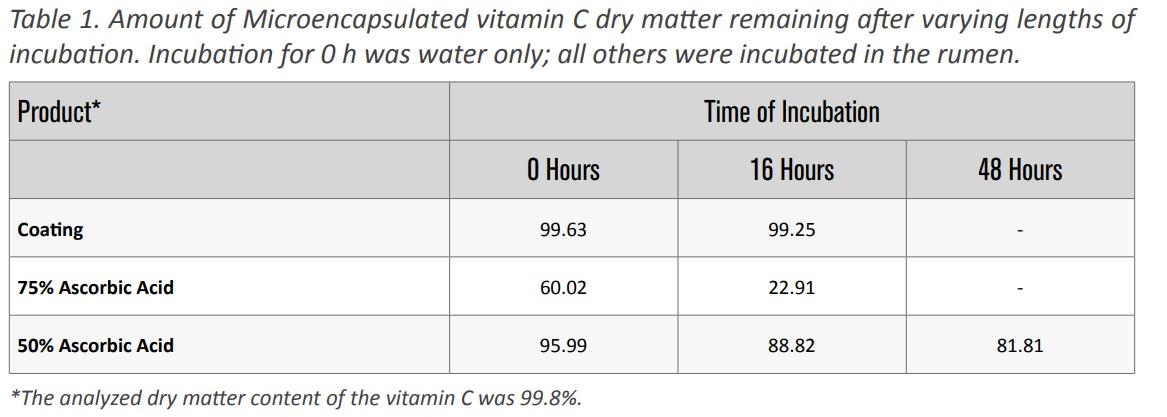
The coating material was not filtered or washed out of the in situ bags during the soaking or washing procedures. The coating also showed evidence of only minimal ruminal degradation after 16 h of in situ ruminal incubation (Table 1).
About 89% of the 50% ascorbic product was not degraded in the rumen after 16 hours of incubation. This means that 89% of the 50% ascorbic acid was available to flow into the lower gut. However, since these are dry matter disappearances, we do not know the exact level of bypass of each nutrient. The worst case scenario is that all of the dry matter disappearance is the coated nutrient (ascorbic acid). Which gives a bypass of about 78% of the ascorbic acid. This compares favorably with other work done with coated microencapsulated vitamin C that showed a rumen bypass of 72%.
The 75% ascorbic acid product had very poor bypass characteristics with only 22.9% of the dry matter remaining after 16 h of in situ ruminal incubation. However, it is important to know that the product is very fine and a substantial amount will escape the in situ bag just because the particles are physically too small to be retained.
A final incubation for 48 h was conducted for the Maxx Performance microencapsulated 50% Vitamin C, the product showing substantial ruminal bypass. This procedure was conducted to provide some information about availability of the product after 16 hours of ruminal incubation. For the 50% ascorbic acid product, a greater ruminal escape was observed after 48 hours with approximately 68% of the ascorbic acid estimated to remain in the rumen. These results suggest that microencapsulation is an effective delivery system to deliver nutrients post-ruminally into the lower gastrointestinal tract. However, the extent of delivery is dependent on particle size.
To more accurately determine the fate of the fine ascorbic acid product particles, an in vitro ruminal evaluation was conducted using the Tilley-Terry method. Three ascorbic acid products (50%, 70%, and 75% ascorbic acid) were evaluated in triplicate for 24 hours. After the in vitro ruminal incubation, the contents of each fermentation tube were filtered through Whatman qualitative filter paper. This paper captured 99% of the products prior to fermentation. Dry matter bypass after the 24 hr in vitro ruminal fermentation was 66.28%, 52.42%, and 52.44% for the 50%, 70%, and 75% ascorbic acid products, respectively. Note that the particle size distribution of the 70% and 75% were the same.
Conclusion
These on farm trial results indicate that microencapsulation of vitamin C is a convenient cost effective method to deliver Vitamin C post-ruminally through rumen protection of this essential nutrient. Encapsulated Vitamin C is a very potent form of ascorbic acid which is a powerful reducing agent (electron donor). It participates in intracellular and extracellular quenching of reactive oxidants, recycling of vitamin E (electron transfer to oxidized tocopherols and tocotrienols), serves as a cofactor in 8 major essential enzymatic reactions, prevents LDL oxidation, and promotes iron absorption in the gastrointestinal tract. Vitamin C is more than an antioxidant but participates in a multitude of essential reactions of life. It is recommended that microencapsulated vitamin C be supplemented to reduce the stress of production in ruminant animals. Microencapsulation is a technology that is available to deliver nutrients post-ruminally into the lower gastrointestinal tract. However, the extent of delivery is dependent on particle size. Additionally, the technology has wide application as a nutrient delivery system across other species especially in simple stomach animals. Microencapsulation represents a cost effective and convenient method to deliver vitamin C post ruminally and to make this nutrient available for use in the lower gut. By microencapsulation, vitamin C is also physically protected from air, light and metals, thus maintaining its potency







.jpg&w=3840&q=75)