Biosynthesis and Characterization of Silver Nanoparticles from Marine Macroscopic Brown Seaweed Colpomenia sinuosa (Mertens ex Roth) Derbes and Solier
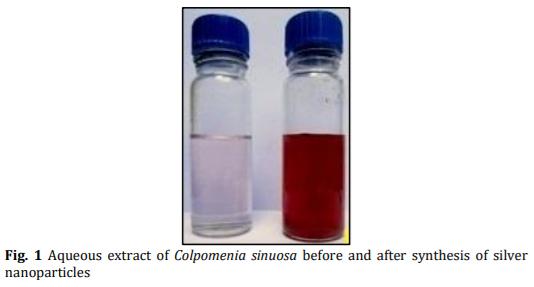
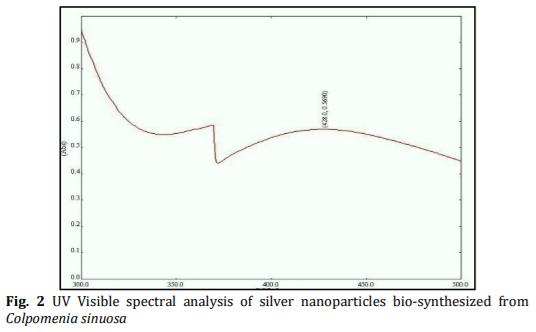
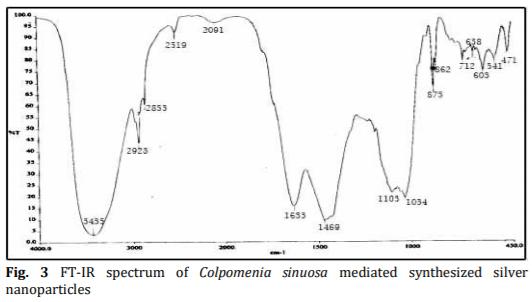
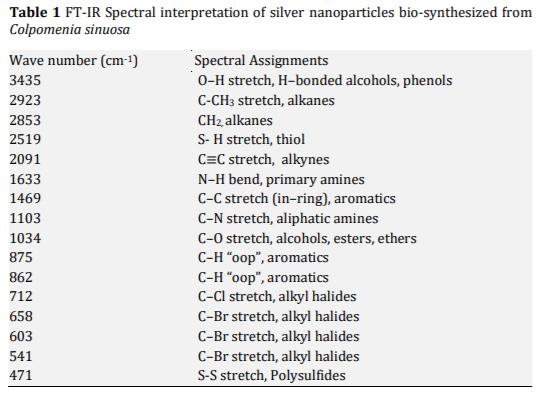
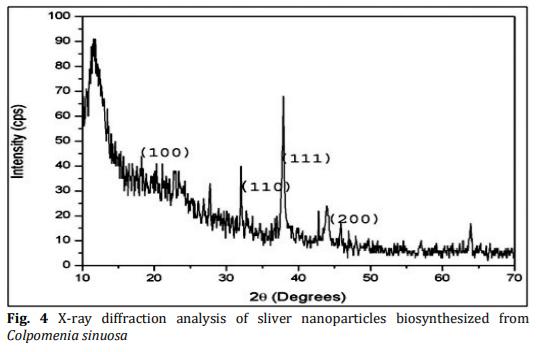
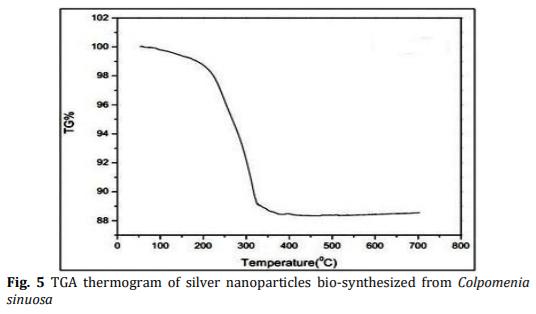
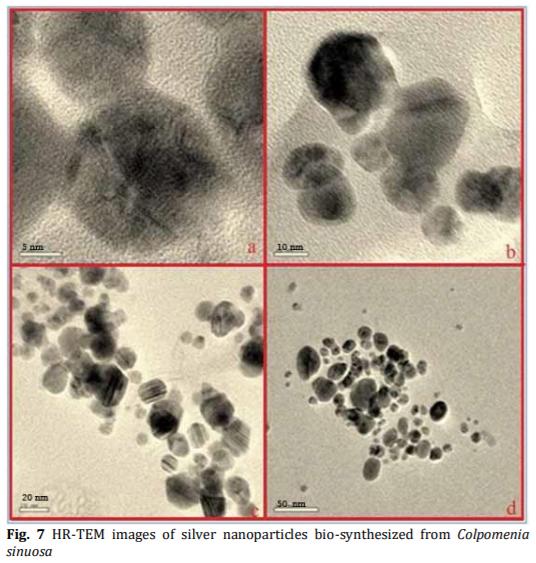
In the present study energy-efficient, economically scalable colloidal silver (Ag) nanoparticles were biosynthesized from marine brown seaweed Colpomenia sinuosa by green synthesis method. The marine macroscopic brown seaweed Colpomenia sinuosa was used in the experimental study for the biosynthesis of silver nanoparticle since they are rich in phytochemicals, bioactive compounds and secondary metabolites which has reducing agents that may be environmentally acceptable and ecofriendly. The biosynthesized silver nanoparticles from marine macroscopic brown seaweed were characterized by UV-vis spectroscopy which confirmed the surface plasmon resonance of silver nanoparticles, Fourier transform infrared (FT-IR) spectroscopy to identify the presence of various functional groups in biomolecules responsible for the bioreduction of Ag+ and capping/stabilization of silver nanoparticles. X-ray diffraction (XRD) to observe face center cubic (fcc) and crystalline nature of silver nanoparticles, thermogravimetric analysis (TGA) which revealed the thermal stability and purity of the silver nanoparticles. Particle size distribution and morphology were investigated by scanning electron microscope (SEM) which showed silver nanoparticles in the size range of 54-85 nm. The particle distribution under different nanometers was analyzed using transmission electron microscopy (TEM).
Keywords: Silver Nanoparticles Biological Green Synthesis Colpomenia sinuosa.

[1] J.L. West, N.J. Halas, Engineered nanomaterials for biophotonics applications: Improving sensing, imaging, and therapeutics, Annu. Rev. Biomed. Eng. 5 (2003) 285-292.
[2] G.F. Paciotti, L. Myer, D. Weinreich, D. Goia, N. Pavel, R.E. McLaughlin, L. Tamarkin, Colloidal gold: A novel nanoparticle vector for tumour directed drug delivery, Drug Delivery 11 (2004) 169-183.
[3] K.K. Jain,Nanotechnology-based drug delivery for cancer, Technol. Cancer. Res. Treat. 4 (2005) 407-16.
[4] X. Wu,H. Liu, J. Liu, K.N. Haley, J.A. Treadway, et al., Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots, Nat. Biotechnol. 21(1) (2003) 41-46.
[5] W.C.W. Chan, D.J. Maxwell, X. Gao, R.E. Bailey, M. Han, S. Nie, Luminescent quantum dots for multiplexed biological detection and imaging, Curr. Opin. Biotechnol. 13(1) (2002) 40-46.
[6] K. Sokolov, M. Follen, J. Aaron, I. Pavlova, A. Malpica, R. Lotan, K.R. Richartz, Real-time vital optical imaging of precancerous drug using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles, Cancer Res. 63 (2003) 1999-2004.
[7] A.P. Alivisatos, The use of nano crystals in biological detection, Nat. Biotechnol. 22(1) (2004) 47-52.
[8] L.R. Hirsch, R.J. Stafford, J.A. Bankson, S.R. Sershen, B. Rivera, et al., Nano shellmediated near-infrared thermal therapy of tumours under magnetic resonance guidance, Proc. Natl. Acad. Sci. 100(23) (2003) 13549-54.
[9] C.M. Niemeyer,Nanoparticles, proteins, and nucleic acids: Biotechnology meets materials science, Angew. Chem. Int. Edn. 40 (2001) 4128-4158.
[10] National Nanotechnology Initiative, What is nanotechnology? http://www.nano.gov/html/facts/whatIsNano.html (Accessed on: 02.08.2008)
[11] G.I. Arun, A.N. Chaudhari, Biogenic synthesis of nano particles and potential applications: An eco-friendly approach, J. Nanomed. Nanotech. 4(2) (2013) 1- 7.
[12] A.V. Thirumalai, D. Prabhu, M.J. Soniya, Stable silver nano particle synthesizing methods and its applications, Biosci. Res. 1(4) (2010) 259-270.
[13] Z.J. Jiang, C.Y. Liu, L.W. Sun, Catalytic properties of silver nanoparticles supported on silica spheres, J. Phys. Chem. B 109 (5) (2005) 1730–1735.
[14] B.P. Rand, P. Peumans, S.R. Forrest, Long-range absorption enhancement in organic tandem thin-film solar cells containing silver nano clusters, J. Appl. Phys. 96 (2004) 7519-7526.
[15] J.R. Cole, N.J. Halas, Optimized plasmonic nano particle distributions for solar spectrum harvesting, Appl. Phys. Lett. 89 (2006) 153120:1-3.
[16] H.J. Zhai, D.W. Sun, H.S. Wang, Catalytic properties of silica/silver nanocomposites, J. Nanosci. Nanotechnol. 6 (2006) 1968-1972.
[17] S. Yamamoto, H. Watarai, Surface-enhanced Raman spectroscopy of dodecanethiol-bound silver nanoparticles at the liquid/liquid interface, Langmuir 22 (2006) 6562-6569.
[18] N. Savage, M.S. Diallo, Nanomaterials and water purification: opportunities and challenges, J. Nanoparticle. Res. 7 (2005) 331-342.
[19] V. Samby, M.M. MacBride, B.R. Peterson, Silver bromide nanoparticle/polymer composites: dual action tunable antimicrobial materials, J. Am. Chem. Soc. 128 (2006) 9798–9808.
[20] S. Pal, Y.K. Tak, J.M. Song, Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli, Appl. Environ. Microbiol. 73 (2007) 1712-1720.
[21] A.D. Maynard, E. Michelson, The nanotechnology consumer products inventory, Woodrow Wilson International Center for Scholars, Http://www.Nanotechproject.Org/44 (Accessed on: 29.05.2007)
[22] M. Fayaz, K. Balaji, M. Girilal, R. Yadav, P.T. Kalaichelvan, R. Venketesan, Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram-negative bacteria, Nanomed. Nanotechnol. Biol. Med. 6(1) (2010) 103-109.
[23] P.C. Lee, D. Meisel, Adsorption and surface enhanced Raman of dyes on silver and gold sols, J. Phys. Chem. 86 (1982) 3391-3395.
[24] H.M.E. Azzazy, M.M.H. Mansour, S.C. Kazmierczak, From diagnostics to therapy: Prospects of quantum dots, Clin. Biochem. 40 (2007) 917-927.
[25] A. Zajac, D. Song, W. Qian, T. Zhukov, Protein microarrays and quantum dot probes for early cancer detection, Colloid. Surf. B 58(2) (2007) 309-314.
[26] K. Kerman, T. Endo, M. Tsukamoto, M. Chikae, Y. Takamura, E. Tamiya, Quantum dot-based immunosensor for the detection of prostate-specific antigen using fluorescence microscopy, Talanta 71(4) (2007) 1494-1499.
[27] K. Sokolov, D. Nida, M. Descour, A. Lacy, M. Levy, et al., Molecular optical imaging of therapeutic targets of cancer, Adv. Cancer Res. 96 (2006) 299–344.
[28] D.L. Nida, M.S. Rahman, K.D. Carlson, R. Richards-Kortum, M. Follen, Fluorescent nanocrystals for use in early cervical cancer detection, Gynecol. Oncol. 99(3) (2005) 89-94.
[29] P. Rajasulochana, R. Dhamotharan, P. Murugakoothan, S. Murugesan, P. Krishnamoorthy, Biosynthesis and characterization of gold nanoparticles using the alga Kappaphycus alvarezii, Int. J. Nanosci. 9(5) (2010) 511-519.
[30] S. Swaminathan, S. Murugesan, S. Damodarkumar, R. Dhamotharan, S. Bhuvaneswari, Synthesis and characterization of gold nano particles from alga Acanthophora specifera (VAHL) Boergesen, Int. J. Nanosci. Nanotech. 2(2) (2011) 85-94.
[31] R. Dhamotharan, D. Punitha, S. Murugesan, T.S. Subha, Brown algal biomass mediated biosynthesis of gold nanoparticles, Int. J. Nanosci. Nanotech. 1(1) (2010) 37-44.
[32] D. Radhika, C. Veerabahu, L. Sakthibama, S. Murugesan, Green synthesis of gold nanoparticles by the marine alga Stoechospermum marginatum, Int. J. Nanotech. Appl. 6(91) (2012) 61-70.
[33] M. Vishnu Kiran, S. Murugesan, Bio-synthesis of silver nano particles from marine alga Halymenia poryphyroides and its antibacterial efficacy, Int. J. Curr. Microbiol. App. Sci. 3(4) (2014) 1-7.
[34] K.S. Rajesh, C. Malrakodi, K.S. Venkat, Synthesis and characterization of silver nanoparticles from marine brown seaweed and its antifungal efficiency against clinical fungal pathogens, Asian. J. Pharm. Clin. Res. 10(2) (2017) 190-193.
[35] P. Mukherjee, A. Ahmad, D. Mandal, S. Senapati, S.R. Sainkar, M.I. Khan, Fungusmediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: A novel biological approach to nanoparticle synthesis, Nano. Lett. 1(10) (2001) 515- 519.
[36] P. Mukherjee, A. Ahmad, D. Mandal, S. Senapati, S.R. Sainkar, M.I. Khan, R. Kumar, Extracellular synthesis of gold nanoparticles by the fungus Fusarium oxysporum, Chem. Biochem. 3(5) (2002) 461- 463.
[37] J. Gonzalo, R. Serna, J. Sol, D. Babonneau, C.N. Afonso, Morphological and interaction effects on the surface plasmon resonance of metal nanoparticles, J. Phys. Condens. Matter 15(42) (2003) 3001-3002.
[38] S. Zavoi, F. Fetea, F. Ranga, R. Pop, A. Baciu, C. Socaciu, Comparative fingerprint and extraction yield of medicinal herb phenolics with hepatoprotective potential, as determined by UV-Vis and FT-MIR spectroscopy, Not. Bot. Horti Agrobot. Cluj-Napoca. 39 (2011) 82-89.
[39] M. Mirderikvandi, A. Kiani, M. Khaldari, M. Alirezae, Effects of artichoke (Cynara scolymus L.) extract on antioxidant status in chicken thigh meat, Iran. J. Vet. Med. 10 (2016) 73-81.
[40] Q. Chen, G. Liu, G. Chen, T. Mi, J. Tai, Green synthesis of silver nanoparticles with glucose for conductivity enhancement of conductive ink, Bioresources 12 (2016) 608-621.
[41] K.L. Niraimathi, R. Lavanya, S. Veerappan, P. Brindha, Green synthesis and characterization of silver nanoparticles from aqueous extract of Basella alba and their invitro antioxidant potentials, Int. J. Pharm. Pharm. Sci. 6 (2014) 393- 396.








