Control of fertility in turkeys: the impact of environment, nutrition and artificial insemination technology
The turkey industry has long taken advantage of the potential offered by artificial insemination, a strategic tool to select male and female lines, and also to optimize the production of broiler chicks.
Indeed, artificial insemination in breeder turkeys has replaced natural mating for over 50 years as it virtually suppresses sexual behaviour constraints in the selection of male and female lines, facilitates high reproductive performance, and ultimately allows permanent optimization of the genetic potential from the best sires.
In practice, adequate management of artificially inseminated flocks is only possible if male and female breeders are subjected to appropriate environmental conditions. For example, controlling the onset and duration of the reproductive period in both sexes requires specific light schedules and feed regimes, which themselves justify specific housing equipment.
Also, artificial insemination can only be successful with high initial quality semen output and appropriate handling procedures to sustain its fertilizing potential. The aim of this text is to highlight some aspects of breeder and semen management known to ultimately exert their action on egg fertility during the reproductive season.
Environmental conditions to sustain fertility in breeder flocks
Modern strains of commercial turkeys and meat type chickens have been primarily selected on the basis of growth rate, feed conversion and meat yield. Unfortunately, this has engendered a series of negative effects on reproductive performance, including early but generally limited persistence of sexual maturity and declining egg fertility (Krueger, 1990; Brillard, 2004).
Early studies in wild turkeys indicated that this species expresses cyclic variations of its reproductive season under natural conditions. More precisely, sexual maturity develops in spring (increasing, followed by longer day length) while winter (decreasing, then short day length) is a period of sexual rest.
In past decades, the natural photoperiodic sensitivity of this species has been profitably monitored by the turkey industry to facilitate the production of turkey chicks irrespective of the natural breeding season. Thus, light regimes specifically adapted to each sex have progressively become available, facilitating the control of the onset and duration of the reproductive period.
A major consequence of the longer reproductive period in females used in Europe has been the need for breeder males capable of sustained, persistent production of semen.
Unfortunately in a majority of cases, a relatively long reproductive season in males has been accompanied by the occurrence of a marked decrease of semen output during the last weeks of the season.
These observations therefore justify the need for additional research devoted to 1) better comprehending testicular development in relation to the environment and age of turkey breeder males, and 2) optimizing management conditions in heavy strains of breeder females.
In males, previous research had demonstrated that declining semen output over the last stages of the reproductive season is the consequence of birds becoming photorefractory, a physiological response to light stimulation in which birds at first stimulated by increasing and/or long photoperiods become progressively refractory to light stimulation. In turkey males as in other poultry species, photorefractory birds show a progressive decline in plasma LH (Follet and Robinson, 1980) accompanied by a non-reversible testicular regression (Godden and Scanes, 1977; Krueger et al., 1977).
Meanwhile, preliminary observations in our laboratory indicate that male turkeys subjected to a moderately short photoperiod (10.5 L) develop early and persistent development of testes over the entire reproductive season.
Besides photoperiod, a factor with a specific role on the reproductive physiology in breeder flocks, daily feed allowance, also plays a major role by acting as a regulator of body (and therefore gonadal) development. Indeed, current management practice in breeder flocks includes precise adjustment of feed allowance depending on genetic origin and sex. A general goal in males is to prevent excessive weight gain, a major cause of poor quality ejaculate and at extremes, early testicular regression.
In turkey breeder females, broodiness expression is a major factor contributing to the limitation of laying performance. This behavioral trait has been counter-selected in several poultry species but is still expressed at a relatively high degree in breeder turkeys.
Recently, new research projects have been undertaken in order to identify broodinessrelated molecular markers, a prerequisite to a rapid selection against this trait.
Accessing and preserving sperm with a high fertilizing potential
Females in avian species share with other females (reptiles, hymenoptera) the ability to store spermatozoa for prolonged periods in specialized structures of the oviduct called sperm storage tubules (Figure 1) located in the uterovaginal junction and in the infundibulum.
Upon selection and storage, sperm are progressively released from the storage sites and then transported to the infundibulum, the site of fertilization of the oocyte (see Bakst et al., 1994, for review). Depending on species and individuals, avian females may therefore lay several fertile eggs after a single mating or insemination, thus defining the so-called ‘duration of fertile period’, which is the number of days during which a given female lays fertile eggs after a single deposition of sperm in the oviduct.
In the turkey, duration of the fertile period may reach up to 8-10 weeks, but the chances of each egg being fertilized progressively decline as time after semen deposition increases (Lorenz, 1950; McCartney, 1951).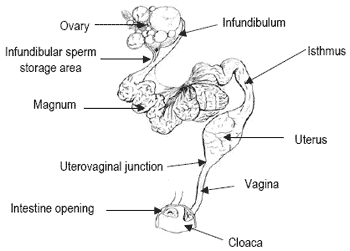
Figure 1. Diagrammatic representation of the avian oviduct (adapted from Brèque et al., 2003).
In addition, semen collection frequency exerts a direct effect on sperm viability, an indication that aging processes of spermatozoa rapidly develop in the vas deferens prior to ejaculation (Table 1). Besides these aspects, procedures to handle semen in vitro along with sperm storage conditions in the oviduct have a permanent major impact on subsequent fertility and hatchability of eggs.
In recent years, extensive research has focused on maintaining structural and functional integrity of sperm membranes in poultry with special emphasis in turkeys and chickens.
In the turkey, studies on the lipid composition of plasma membranes indicated high concentrations of polyunsaturated fatty acids (PUFAs) and age-dependent changes in the PUFA composition of these membranes (Douard et al., 2003). Previously cited work also demonstrated that membrane susceptibility to lipid peroxidation is higher in fresh or stored (48 hr) ejaculate collected from older compared to young turkey breeder males.
Table 1. Incidence of semen collection frequency on subsequent sperm viability in the turkey.
Noirault and Brillard, 1999
abcdValues with different superscripts differ (P<0.05)
In order to counteract such detrimental effects of age on sperm viability and motility, two approaches have been proposed. One is based on the addition of antioxidants in the diluents during in vitro storage and the other involves dietary antioxidant supplementation of breeder flocks. As antioxidants, vitamin E and selenium (as selenoenzymes) are known to prevent lipid oxidation as described by Surai (1999, Figure 2).
Several studies have confirmed that in vitro supplementation of diluents with vitamin E favors maintenance of sperm motility and viability, a prerequisite to in vivo sperm storage in the oviduct (Douard et al., 2004).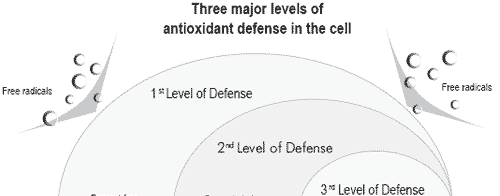
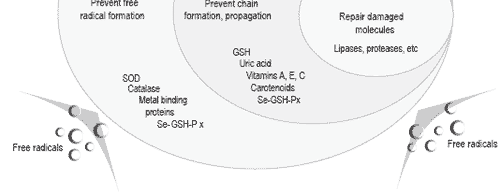
Figure 2. A schematic representation of antioxidant defenses of the cell against lipid peroxidation (from Surai, 1999).
Besides in vitro approaches, attempts have been made to enhance antioxidant defenses either directly in the male tract or indirectly through the micro-environment of oviductal sperm storage sites by dietary supplementation of chicken or turkey breeder diets with antioxidants.
In addition to vitamin E, dietary selenium supplementation was also attempted as selenium deficiency leads to alteration of spermatogenesis, impaired motility of spermatozoa and morphological alteration of the sperm midpiece. A positive influence of organic selenium (Sel-Plex®, Alltech Inc.) on prolonged sperm storage in the female oviduct and on egg fertility was, for example, demonstrated in the chicken and is likely to also occur in other poultry species including turkeys (Breque et al., 2003).
Besides fertility, observations in chicken and turkey embryos also revealed that organic selenium (Sel-Plex®) and vitamin E supplementation of the maternal diet increase Se-dependent glutathione peroxidase (GSH-Px) activity in the liver of hatched chicks (chicken) and helps maintain it at high levels, which reduces oxidative stress and optimizes regulation of proteins specialized in the protection of embryos during and after heat stress (turkeys).
Sperm-egg interaction: an in vitro approach to better assess fertility in vivo
Fertility assessment of eggs in all poultry species is commonly performed by means of egg candling, a technique in which the presence or absence of embryo development in incubated eggs is revealed to the naked eye of the observer by checking the transparency of eggs lighted with a powerful source. The technique is simple and can be automated, but cannot allow estimation of the population of spermatozoa that may have interacted with the egg in the infundibulum, the site of fertilization.
In contrast, techniques to quantify either directly the population of spermatozoa present in the outer perivitelline layer of an egg (OPVL-sperm) or indirectly by estimating the number of holes made by enzymatic digestion in the inner perivitelline layer (IPVL-holes) provide quantitative information on the actual condition of sperm storage in the hen’s oviduct (see review by Wishart, 1997).
Recently, Hazary and Wishart (2004) reported a series of experiments in which they demonstrated that the frequency of IPVL-holes in the germinal disk of turkey eggs provides sensitive and predictive information on flock fertility well before egg fertility starts declining. Such observations, which require a standard darkfield microscope along with some training, are inexpensive compared to the costs engendered by fertility losses in large size flocks of breeders. Furthermore, they provide rapid (<24 hrs) information to poultry (turkey) managers to help them decide whether or not increased semen doses, additional artificial inseminations or even replacement of males, are required.
| Conclusion In recent years, available knowledge in the field of turkey reproduction has brought new insight to decision makers willing to optimize management techniques in breeder flocks. Thus, questions such as “How often should we milk toms?” or “What percentage of males should we keep to maintain fertility?” may no longer be treated separately, as they are obviously directly linked. In addition, some techniques have become available to anticipate fertility decline in aging flocks. Such techniques may appear long and tedious. On the other hand, they offer new possibilities for optimizing egg fertility over the reproductive season. It remains that in turkeys, as in other poultry species, high fertility can only be achieved if male and female gametes are themselves of high initial quality. Defining and assessing gamete quality in both sexes is therefore a new challenge in poultry species, and especially in turkeys. Prevention of oxidative stress caused by inappropriate environment of gametes and embryos can be sustained by adequate supplementation of antioxidants to the diet both in male and female breeder turkeys. |
References
Bakst, M.R, G. Wishart and J.P. Brillard. 1994. Oviductal sperm selection, transport, and storage in poultry. Poult. Sci. Rev. 5:117-143.
Brèque, C., P. Surai and J.P. Brillard. 2003. Roles of antioxidants in prolonged storage of avian spermatozoa in vivo and in vitro. Molecular Reprod. Dev. 66:314-323.
Brillard, J.P. 2004. Natural mating in broiler breeders: present and future concerns. World’s Poult. Sci. J. 60:439-445.
Douard, V., D. Hermier, M. Magistrini and E. Blesbois. 2003. Reproductive period affects lipid composition and quality of fresh and stored spermatozoa in turkeys. Theriogenology 59:753-764.
Douard, V., D. Hermier, M. Magistrini, C. Labbé and E. Blesbois. 2004. Impact of changes in composition of storage medium on lipid content and quality of turkey spermatozoa. Theriogenology 61:1-13.
Follet, B.K. and J.E. Robinson. 1980. Photoperiod and gonadotrophin secretion in birds. Prog. Reprod. Biol. 5:39-61.
Godden, P.M.M. and C.G. Scanes. 1977. Gonado trophin concentrations during growth and maturation in domestic turkeys. Brit. Poult. Sci. 18:675-685.
Hazary, R.C. and G.J. Wishart. 2004. Studies on sperm-egg interaction to evaluate reproductive efficiency in turkey. In: Proceedings of the 22nd World’s Poultry Congress. Istanbul, Turkey, 8-13 June.
Krueger, K.K.K. 1990. Fertility in female turkeys: How to manage it? In: Control of Fertility in Domestic Birds (J.P. Brillard, ed). INRA-Tours, Paris, France, pp. 205- 212.
Krueger, K.K.K, J.A. Owen, C.E. Kruegern and T.M. Ferguson. 1977. Effect of feed or light restriction during the growing and breeding cycles on the reproductive performance of broad breasted white turkey males. Poult. Sci. 56:1566-1574.
Lorenz, F.W. 1950. Onset and duration of fertility in turkeys. Poult. Sci. 29:20-26.
McCartney, M.G. 1951. The physiology of reproduction in turkeys. 2. Degree and duration of fertility and hatchability in broody and non-broody pullets. Poult. Sci. 30:663-667.
Noirault, J., and J.P. Brillard. 1999. Effects of frequency of semen collection on quantitative and qualitative characteristics of semen in turkey breeder males. Poult. Sci. 78:1034-1039.
Surai, P.F. 1999. Vitamin E in avian reproduction. Poult. Avian Bio. Rev. 10:1-60.
Wishart, J.G. 1997. Quantitative aspects of sperm:egg interactions in chickens and turkeys. Anim. Reprod. Sci. 48:81-89.








